Rituximab
Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer.[18] It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia (in children and adults, but not recommended in elderly patients), rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers.[16] Severe side effects include reactivation of hepatitis B in those previously infected, progressive multifocal leukoencephalopathy, toxic epidermal necrolysis, and death.[9][18] Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells.[27] In the United States, rituximab is indicated to treat: In the European Union, rituximab is indicated for the treatment of follicular lymphoma and diffuse large B cell non-Hodgkin's lymphoma (two types of non-Hodgkin's lymphoma, a blood cancer);[16] chronic lymphocytic leukemia (CLL, another blood cancer affecting white blood cells);[16] severe rheumatoid arthritis (an inflammatory condition of the joints);[16] two inflammatory conditions of blood vessels known as granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA);[16] moderate to severe pemphigus vulgaris, an autoimmune disease characterised by widespread blistering and erosion of the skin and mucous membranes (the linings of internal organs).[32] In the United States, it has been FDA approved for use in combination with methotrexate for reducing signs and symptoms in adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to one or more anti-TNF-alpha therapy.[36][37] The most dangerous, although among the most rare, side effect is progressive multifocal leukoencephalopathy infection, which is usually fatal; however, only a very small number of cases have been recorded occurring in autoimmune diseases.[49] Serious adverse events, which can cause death and disability, include:[15][18] A concern with continuous rituximab treatment is the difficulty to induce a proper vaccine response.This was brought into focus during the COVID-19 pandemic, where persons with multiple sclerosis and rituximab treatment had higher risk of severe COVID-19.Although the function of CD20 is unknown, it may play a role in Ca2+ influx across plasma membranes, maintaining intracellular Ca2+ concentration and allowing activation of B cells.[65] Based on its safety and effectiveness in clinical trials,[66] rituximab was approved by the US Food and Drug Administration (FDA) in 1997 to treat B-cell non-Hodgkin lymphomas resistant to other chemotherapy regimens.[92] In 2009, a patient receiving methotrexate-induced B-cell depletion for cancer treatment, experienced a transient remittal of their myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptoms.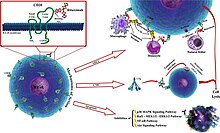

Monoclonal antibodyChimericTargetBiosimilarsDrugs.comMedlinePlusLicense dataDailyMedPregnancycategoryRoutes ofadministrationIntravenousDrug classATC codeL01FA01Legal status ℞-onlyWARNINGPharmacokineticBioavailabilityElimination half-lifeExcretionCAS NumberDrugBankChemSpiderChEMBLCompTox DashboardECHA InfoCardFormulaMolar massautoimmune diseasescancernon-Hodgkin lymphomachronic lymphocytic leukemiarheumatoid arthritisgranulomatosis with polyangiitisidiopathic thrombocytopenic purpurapemphigus vulgarismyasthenia gravisEpstein–Barr virusmucocutaneous ulcersintravenous infusionhepatitis Bprogressive multifocal leukoencephalopathytoxic epidermal necrolysispregnancyB cellsWorld Health Organization's List of Essential MedicinesBiogenGenentechChugai Pharmaceuticalssurfaceantigenplasma cellshematopoietic stem cellsindicatedTNF inhibitorsvasculitidesmicroscopic polyangiitisdiffuse large B-cell lymphomaBurkitt lymphomaB-cell leukemiacancers of the white blood systemleukemiaslymphomasWaldenström's macroglobulinemiahyaluronidasefollicular lymphomafludarabinecyclophosphamiderandomised controlled trialsrefractoryFDA approvedmethotrexateTNF-alphasafetyoff-labelmultiple sclerosissystemic lupus erythematosuschronic inflammatory demyelinating polyneuropathyanemiasautoimmune hemolytic anemiapure red cell aplasiathrombotic thrombocytopenic purpuraEvans syndromebullouspemphiguspemphigoiddiabetes mellitusSjögren syndromeanti-NMDA receptor encephalitisDevic's diseaseAnti-AQP4 diseaseMOG antibody diseaseGraves' ophthalmopathyautoimmune pancreatitisOpsoclonus myoclonus syndromeIgG4-related diseaseCardiac arrestCytokine release syndromeTumor lysis syndromeacute kidney injuryJC viruslymphomaPulmonaryBowel obstructionperforationCOVID-19 pandemicPfizer–BioNTech COVID-19 vaccinedifferentiationantibody-dependent cellular cytotoxicitycomplement-dependent cytotoxicitycell cycleMHC IIB cell receptorapoptosisstem cellsamino acidsdisulfide bondIDEC Pharmaceuticalsclinical trialsFood and Drug Administrationnon-Hodgkin lymphomaschemotherapyCHOP chemotherapyEuropean CommissionB-cell CLL/lymphoma