Apoptosis
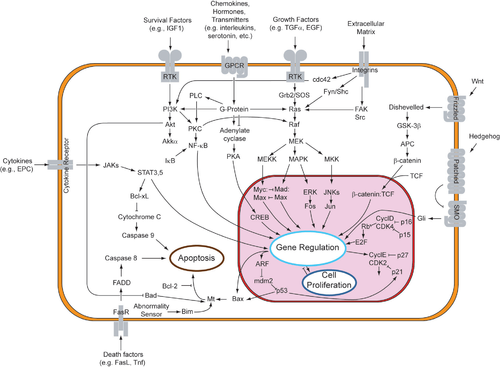
[4] In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle.While studying tissues using electron microscopy, John Kerr at the University of Queensland was able to distinguish apoptosis from traumatic cell death.Kerr, Wyllie and Currie credited James Cormack, a professor of Greek language at University of Aberdeen, with suggesting the term apoptosis.[12] The 2002 Nobel Prize in Medicine was awarded to Sydney Brenner, H. Robert Horvitz and John Sulston for their work identifying genes that control apoptosis.[15][17] In English, the p of the Greek -pt- consonant cluster is typically silent at the beginning of a word (e.g. pterodactyl, Ptolemy), but articulated when used in combining forms preceded by a vowel, as in helicopter or the orders of insects: diptera, lepidoptera, etc.In the original Kerr, Wyllie & Currie paper,[10] there is a footnote regarding the pronunciation: We are most grateful to Professor James Cormack of the Department of Greek, University of Aberdeen, for suggesting this term.[19] The intrinsic pathway is activated by intracellular signals generated when cells are stressed and depends on the release of proteins from the intermembrane space of mitochondria.[20] The extrinsic pathway is activated by extracellular ligands binding to cell-surface death receptors, which leads to the formation of the death-inducing signaling complex (DISC).[28][29] Before the actual process of cell death is precipitated by enzymes, apoptotic signals must cause regulatory proteins to initiate the apoptosis pathway.[23][32] There is also a growing body of evidence indicating that nitric oxide is able to induce apoptosis by helping to dissipate the membrane potential of mitochondria and therefore make it more permeable.IAP also normally suppresses the activity of a group of cysteine proteases called caspases,[37] which carry out the degradation of the cell.[39] Binding of this receptor can also indirectly lead to the activation of transcription factors involved in cell survival and inflammatory responses.[42] Luminescent iridium complex-peptide hybrids (IPHs) have recently been designed, which mimic TRAIL and bind to death receptors on cancer cells, thereby inducing their apoptosis.[44] Following TNF-R1 and Fas activation in mammalian cells[citation needed] a balance between proapoptotic (BAX,[45] BID, BAK, or BAD) and anti-apoptotic (Bcl-Xl and Bcl-2) members of the Bcl-2 family are established.The proapoptotic homodimers are required to make the mitochondrial membrane permeable for the release of caspase activators such as cytochrome c and SMAC.[75] This exon encodes a portion of the mature TNF domain, as well as the leader sequence, which is a highly conserved region necessary for proper intracellular processing.When an APAF-1 gene trap is introduced into cells, many morphological changes occur, such as spina bifida, the persistence of interdigital webs, and open brain.Finally, a caspase 3 knock-out was characterized by ectopic cell masses in the brain and abnormal apoptotic features such as membrane blebbing or nuclear fragmentation [citation needed].As a consequence, the balance of anti-apoptotic and proapoptotic effectors is upset in favour of the former, and the damaged cells continue to replicate despite being directed to die.Part of this pathway includes alpha-interferon and beta-interferon, which induce transcription of the p53 gene, resulting in the increase of p53 protein level and enhancement of cancer cell-apoptosis.Some apoptotic factors are vital during mitochondrial respiration e.g. cytochrome C.[90] Pathological inactivation of apoptosis in cancer cells is correlated with frequent respiratory metabolic shifts toward glycolysis (an observation known as the "Warburg hypothesis".To stimulate apoptosis, one can increase the number of death receptor ligands (such as TNF or TRAIL), antagonize the anti-apoptotic Bcl-2 pathway, or introduce Smac mimetics to inhibit the inhibitor (IAPs).[52] The addition of agents such as Herceptin, Iressa, or Gleevec works to stop cells from cycling and causes apoptosis activation by blocking growth and survival signaling further upstream.[102] The progression of the human immunodeficiency virus infection into AIDS is due primarily to the depletion of CD4+ T-helper lymphocytes in a manner that is too rapid for the body's bone marrow to replenish the cells, leading to a compromised immune system.[105] Researchers from Kumamoto University in Japan have developed a new method to eradicate HIV in viral reservoir cells, named "Lock-in and apoptosis."Using the synthesized compound Heptanoylphosphatidyl L-Inositol Pentakisphophate (or L-Hippo) to bind strongly to the HIV protein PR55Gag, they were able to suppress viral budding.[109] Apoptosis caused by CDV is typically induced via the extrinsic pathway, which activates caspases that disrupt cellular function and eventually leads to the cells death.[92] This change in the caspase cascade suggests CDV induces apoptosis via the intrinsic pathway, excluding the need for the initiator caspase-8.They can be exported in the apoptotic bodies that pinch off from the surface of the dying cell, and the fact that they are engulfed by phagocytes prevents the initiation of a host response.[120] Later studies linked this phenomenon to the release of AIF (apoptosis-inducing factor) from the mitochondria and its translocation into the nucleus mediated by its NLS (nuclear localization signal).






etoposideDU145 prostate cancer celltime-lapse microscopyquantitative phase-contrast microscopyAnatomical terminologyAncient Greekromanizedprogrammed cell deathmulticellular organismsBiochemicalmorphologyblebbingcell shrinkagenuclear fragmentationchromatin condensationDNA fragmentationbillionnecrosisembryophagocytescell stresscaspasesproteasesatrophycancerFas receptorsBcl-2 familyHistory of apoptosis researchCarl VogtWalther FlemmingJohn KerrAlastair CurrieAndrew WyllieUniversity of AberdeenBritish Journal of CancerPaul Ehrlich and Ludwig Darmstaedter PrizeH. Robert HorvitzNobel Prize in MedicineSydney BrennerJohn SulstonC. elegansHippocratesconsonant clusterpterodactylPtolemyhelicopterdipteralepidopteramitochondrialdeath-inducing signaling complexglucocorticoidshypoxiacalciumpoly ADP ribose polymerasemitochondriaadaptor proteinscalpainnitric oxidemembrane potentialcytochrome cApaf-1apoptosomecaspase-9cytosolproteins that inhibit apoptosiscysteine proteasestumor necrosis factorligandTNF-alphacytokinemacrophagestranscription factorsautoimmune diseasesTNF-alpha receptor superfamilyActivation-induced cell deathfas receptortransmembrane proteinFas ligandBcl-Xlhomodimersactivator proteinproteolyticapoptosis-inducing factorXenopus laevisdrug resistanceNF-κBlamellipodianuclear envelopepyknosiskaryorrhexisendonucleaseelectrophoresisDNA ladderingischemicmicrotubulePannexin 1Fragmentationvesiclesphagocytosisefferocytosisphosphatidylserinescramblaseinflammatory responseknock-outslive cell imagingflow fluorocytometrytransmission electron microscopyWestern blottingstainedNCI-H460overexpressedcell lineinterferoncell cycleMiCK assay14-3-3human immunodeficiency virusCD4+ T-helper lymphocytesprotein kinase Rnatural killercytotoxic T cellsCanine distemper virusextrinsic pathwayintrinsic pathwayOropouche virusBunyaviridae