Estradiol sulfate
[2][4][5] Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues.[11] E2S shows about 10,000-fold lower potency in activating the estrogen receptors relative to estradiol in vitro.[12] It is 10-fold less potent than estrone sulfate orally in terms of in vivo uterotrophic effect in rats.[14] This in part led to the introduction of conjugated estrogens (Premarin), which are primarily estrone sulfate, in 1941.[14] Although inactive at steroid hormone receptors, E2S has been found to act as a potent inhibitor of glutathione S-transferase,[15] an enzyme that contributes to the inactivation of estradiol via conversion of it into an estradiol-glutathione conjugate.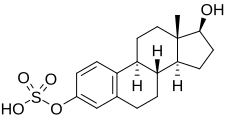


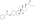
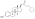
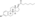
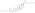







IUPAC nameCAS NumbersodiumChEMBLChemSpiderPubChemCompTox DashboardSMILESChemical formulaMolar massstandard statenaturalendogenousestrogen estersteroid sulfataseestradiolestrogenestrogen sulfotransferasesequilibriumEstronemetabolic sourcesestrone sulfatebreastbreast cancersodium saltconjugated equine estrogensPremarinprodrugmetabolitepharmaceutical drugpotencyestrogen receptorsin vitropotentin vivouterotrophicestrogenicconjugated estrogenssteroid hormone receptorsinhibitorglutathione S-transferaseenzymeglutathioneconjugateovulationEstradiol 3-glucuronideEstradiol 17β-glucuronideEstradiol benzoateEstradiol 17β-acetateEstradiol diacetateEstradiol propionateEstradiol valerateEstradiol cypionateEstradiol palmitateEstradiol stearateEstrone glucuronideEthinylestradiolMestranolQuinestrolRelative binding affinitiesin-vitrolabeledrodentuterinecytosolEstrogen estershydrolyzedhalf-maximal effective concentrationsβ‐galactosidasegreen fluorescent proteinproductionassaysmammalianEstradiol acetateEthanoic acidBenzoic acidEstradiol dipropionatePropanoic acidPentanoic acidEstradiol benzoate butyratebutyric acidCyclopentylpropanoic acidEstradiol enanthateHeptanoic acidEstradiol dienanthateEstradiol undecylateUndecanoic acidOctadecanoic acidEstradiol distearateSulfuric acidEstradiol glucuronideGlucuronic acidEstramustine phosphateNormustinephosphoric acidPolyestradiol phosphatecarbonstraight-chain fatty acidsaromaticcyclicoctanol/water partition coefficientlipophilicityhydrophobicityDrugBankPolymerestradiol phosphaterepeat unitsCatechol estrogenDHEA sulfateEstriol sulfateEstrogen conjugateLipoidal estradiolPregnenolone sulfateHOE2OHvariousHOE2O4PH2(OE2O3PH)nHSO4E2O4SHEstradiol (as a hormone)Estradiol (as a medication)Pharmacodynamics of estradiolPharmacokinetics of estradiolEstrogen (as a hormone)Estrogen (as a medication)Menopausal hormone therapyFeminizing hormone therapy