Polyestradiol phosphate
Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is an estrogen medication which is used primarily in the treatment of prostate cancer in men.[5][2] The safety profile of parenteral estradiol esters like PEP is greatly improved relative to synthetic oral estrogens like ethinylestradiol and diethylstilbestrol.[20][7] PEP has been compared to combined androgen blockade (CAB; castration plus flutamide) for the treatment of prostate cancer in a large randomized clinical trial of 915 patients.[21][22] These findings suggest that parenteral forms of estradiol may have similar effectiveness and safety relative to androgen deprivation therapy (ADT) in the treatment of prostate cancer.[21][22] In addition, estrogens may have significant advantages relative to ADT in terms of bone loss and fractures, hot flashes, sexual function, and quality of life, as well as considerable cost savings with parenteral forms of estradiol compared to GnRH analogue therapy.[24] At a dosage of 160 mg/month, PEP incompletely suppresses testosterone levels, failing to reach the castrate range, and is significantly inferior to orchiectomy in slowing disease progression.[11][19][25][5] PEP is provided in the form of powder or an aqueous solution in vials and ampoules alone or in combination with mepivacaine and/or nicotinamide (vitamin B3) for administration via intramuscular injection.[1] Common or frequent (>10%) side effects are considered to include headache, abdominal pain, nausea, rash, pruritus, loss of libido, erectile dysfunction, breast tenderness, gynecomastia, feminization, demasculinization, infertility, and vaginal bleeding or spotting.[1][32] Side effects that occur occasionally or uncommonly (0.1–1%) include sodium and water retention, edema, hypersensitivity, breast tension, depression, dizziness, visual disturbances, palpitations, dyspepsia, erythema nodosum, urticaria, and chest pain.[1] The rare (<0.1%) side effects of PEP are considered to include weight gain, impaired glucose tolerance, mood changes (elation or depression), nervousness, tiredness, headache, migraine, intolerance of contact lenses, hypertension, thrombosis, thrombophlebitis, thromboembolism, heart failure, myocardial infarction, vomiting, bloating, cholestatic jaundice, cholelithiasis, transient increases in transaminases and bilirubin, erythema multiforme, hyperpigmentation, muscle cramps, dysmenorrhea, vaginal discharge, premenstrual-like symptoms, breast enlargement, testicular atrophy, allergic reactions (e.g., urticaria, bronchial asthma, anaphylactic shock) due to mepivacaine, and injection site reactions (e.g., pain, sterile abscesses, inflammatory infiltrates).[33][34][6] The difference between the two types of therapies is due to the bioidentical and parenteral nature of PEP and its minimal influence on liver protein synthesis.[2] However, PEP at a higher dosage of 240 mg/month has subsequently been found in large studies to significantly increase cardiovascular morbidity relative to GnRH modulators and orchiectomy in men treated with it for prostate cancer.[46] A single intramuscular injection of 320 mg PEP in men has been found to suppress testosterone levels to within the castrate range (< 50 ng/dL) within 3 weeks.[47] Studies have found a markedly increased 5-year risk of cardiovascular mortality of 14 to 26% in men treated with oral synthetic estrogens like ethinylestradiol and diethylstilbestrol for prostate cancer.[7] Another study found no change in levels of coagulation factor VII, a protein of particular importance in the cardiovascular side effects of estrogens, with 240 mg/month intramuscular PEP.[7] Originally, PEP was typically used at a dosage of 80 mg per month in combination with 150 μg per day oral ethinylestradiol in the treatment of prostate cancer.[1] Early studies found that a dosage of 80 mg PEP every 4 weeks rapidly produced relatively high mean estradiol levels of about 400 to 800 pg/mL.[15][69][46][70] It has been determined via ultracentrifugation that the mean molecular weight of PEP corresponds to a chain length of approximately 13 repeat units of estradiol 17β-phosphate.[72] PEP is of very low solubility in water, acetone, chloroform, dioxane, and ethanol, but dissolves readily in bases, especially in aqueous pyridine.[63] When the same scientists wanted to synthesize simple phosphates of phloretin, a compound found in apple tree leaves,[74] they accidentally created a polymer instead.[15][26][1][76][77][14] It is no longer available in the United States, Switzerland, and certain other countries however,[13][16] but is still known to be marketed in Austria, Belgium, Denmark, Finland, the Netherlands, Norway, and Sweden.

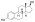
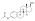

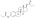

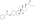
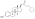
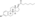








Polyestriol phosphateEstradiol phosphateSkeletal structureball-and-stick modelmonomerDrugs.comPregnancycategoryRoutes ofadministrationIntramuscular injectionDrug classEstrogenEstrogen esterATC codeL02AA02Legal statusPharmacokineticBioavailabilityProtein bindingalbuminMetabolismkidneysgonadsmusclephosphatasesMetabolitesEstradiolphosphoric acidElimination half-lifeExcretionconjugatesIUPAC nameCAS NumberDrugBankChemSpiderChEMBLFormulaMolar massPolymerRepeat unitMelting pointprostate cancerbreast cancerhormone therapylow estrogen levelsmenopausalsymptomsfeminizing hormone therapytransgender womeninjection into muscleside effectsheadachebreast tendernessbreast developmentfeminizationsexual dysfunctioninfertilityvaginal bleedingagonistestrogen receptorbiological targetestrogensprodrugbiological half-lifenaturalbioidenticalsafetyparenteralsyntheticethinylestradioldiethylstilbestrolUnited Statesestradiol undecylateestradiol valerateEuropeestrogen therapypostmenopausalhormone replacement therapyhypogonadismmenopausehigh-dose estrogencombined androgen blockadecastrationflutamideclinical trialsurvivalcardiovascular toxicityeffectivenessandrogen deprivation therapybone lossfractureshot flashessexual functionquality of lifeGnRH analoguegynecomastiaprophylacticirradiationmastectomytestosteroneorchiectomyaqueous solutionampoulesmepivacainenicotinamidevitamin B3local anaestheticburning sensationcontraindicationsHypersensitivityestrogen-dependentmalignant tumorsendometrial cancerendometrial hyperplasiaThrombosisthrombophlebitisvenous thromboembolismdeep vein thrombosispulmonary embolismarterial thromboembolismanginamyocardial infarctionthrombophiliaprotein C deficiencyprotein S deficiencyantithrombin deficiencyarrhythmia