Etiocholanolone glucuronide
Etiocholanolone glucuronide (ETIO-G) is an endogenous, naturally occurring metabolite of testosterone.[1][2] It is formed in the liver from etiocholanolone by UDP-glucuronyltransferases.[1] ETIO-G has much higher water solubility than etiocholanolone and is eventually excreted in the urine via the kidneys.[1][2] Along with androsterone glucuronide, it is one of the major inactive metabolites of testosterone.You can help Wikipedia by expanding it.This biochemistry article is a stub.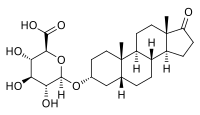
IUPAC nameCAS NumberChemSpiderPubChemCompTox DashboardSMILESChemical formulaMolar massstandard stateendogenousnaturally occurringmetabolitetestosteroneetiocholanoloneUDP-glucuronyltransferaseswater solubilityexcretedkidneysandrosterone glucuronide3α,5β-Androstanediol5β-DihydrotestosteroneAndrostanediol glucuronidesteroidsPrecursorsCholesterol22R-Hydroxycholesterol20α,22R-DihydroxycholesterolPregnenolone17α-Hydroxypregnenolone21-HydroxypregnenoloneCorticosteroidsGlucocorticoids3α,5α-Tetrahydrocorticosterone5α-Dihydrocorticosterone11-Deoxycorticosterone11-Deoxycortisol11-Ketoprogesterone21-Deoxycortisol21-DeoxycortisoneCorticosteroneCortisolCortisone17α-HydroxyprogesteroneProgesterone5α-DihydrocortisolMineralocorticoids5α-Dihydroaldosterone11-Dehydrocorticosterone (11-oxocorticosterone, 17-deoxycortisone)11β-Hydroxyprogesterone (21-deoxycorticosterone)18-Hydroxy-11-deoxycorticosterone18-HydroxycorticosteroneAldosteroneSex steroidsAndrogens11-Ketodihydrotestosterone11-Ketotestosterone7β-Hydroxyepiandrosterone11β-HydroxyandrostenedioneAdrenosterone (11-ketoandrostenedione)AndrostenediolAndrostenedioneAndrosteroneDehydroandrosteroneDHEA sulfateDihydrotestosteroneEpiandrosteroneEpitestosterone16α-Hydroxyandrostenedione16α-Hydroxy-DHEA16α-Hydroxy-DHEA sulfate3α-Androstanediol3α-Androstanediol glucuronide3β-Androstanediol3α-Etiocholanediol3β-EtiocholanediolAndrostenediol sulfateAndrostenetriolAndrosterone sulfateEtiocholanedioneEpietiocholanoloneTestosterone glucuronideTestosterone sulfateEstrogensEstetrolEstradiolEstroneEstriol17α-Estradiol16β-Epiestriol (16β-hydroxyestradiol)17α-Epiestriol (16α-hydroxy-17α-estradiol)16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)2-Hydroxyestradiol2-Hydroxyestriol2-Hydroxyestrone4-Hydroxyestradiol4-Hydroxyestriol4-Hydroxyestrone4-Methoxyestradiol4-Methoxyestrone16α-Hydroxyestrone16β-Hydroxyestrone16-Ketoestradiol16-Ketoestrone27-Hydroxycholesterol4-Androstenedione5-Androstenediol7-Keto-DHEA7α-Hydroxy-DHEA2-Methoxyestradiol2-Methoxyestrone2-Methoxyestriol4-MethoxyestriolEstradiol disulfateEstradiol glucuronideEstradiol 3-glucuronideEstradiol 3-glucuronide 17β-sulfateEstradiol sulfateEstradiol 17β-sulfateEstrone glucuronideEstrone sulfateEstriol glucuronideEstriol sulfateLipoidal estradiolestradiol stearateestradiol palmitateProgestogens