Polyphenol
[4] The term polyphenol is not well-defined, but it is generally agreed that they are natural products with "several hydroxyl groups on aromatic rings" including four principal classes: "phenolic acids, flavonoids, stilbenes, and lignans".The raspberry ellagitannin,[8] on the other hand, with its 14 gallic acid moieties (most in ellagic acid-type components), and more than 40 phenolic hydroxyl groups, meets the criteria of both definitions of a polyphenol.Other examples of compounds that fall under both the WBSSH and Quideau definitions include the black tea theaflavin-3-gallate shown below, and the hydrolyzable tannin, tannic acid.[29] Quantitation results produced by the mean of diode array detector–coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for all polyphenolic molecules.For instance, in the Indian subcontinent, pomegranate peel, high in tannins and other polyphenols, or its juice, is employed in the dyeing of non-synthetic fabrics.[31][32] Natural polyphenols have long been proposed as renewable precursors to produce plastics or resins by polymerization with formaldehyde,[33] as well as adhesives for particleboards.[34] The aims are generally to make use of plant residues from grape, olive (called pomaces), or pecan shells left after processing.These functions include:[41] Flax and Myriophyllum spicatum (a submerged aquatic plant) secrete polyphenols that are involved in allelopathic interactions.[50]Some polyphenols are considered antinutrients – compounds that interfere with the absorption of essential nutrients – especially iron and other metal ions, which may bind to digestive enzymes and other proteins, particularly in ruminants.[52] Polyphenols in wine, beer and various nonalcoholic juice beverages can be removed using finings, substances that are usually added at or near the completion of the processing of brewing.[citation needed] With respect to food and beverages, the cause of astringency is not fully understood, but it is measured chemically as the ability of a substance to precipitate proteins.[60][61] In the European Union, two health claims were authorized between 2012 and 2015: 1) flavanols in cocoa solids at doses exceeding 200 mg per day may contribute to maintenance of vascular elasticity and normal blood flow;[62][63] 2) olive oil polyphenols (5 mg of hydroxytyrosol and its derivatives (e.g. oleuropein complex and tyrosol) may "contribute to the protection of blood lipids from oxidative damage", if consumed daily.[70] In the 1930s, polyphenols (then called vitamin P) were considered as a factor in capillary permeability, followed by various studies through the 21st century of a possible effect on cardiovascular diseases.[78] Phlebotonics of heterogeneous composition, consisting partly of citrus peel extracts (flavonoids, such as hesperidin) and synthetic compounds, are used to treat chronic venous insufficiency and hemorrhoids.[79] Some are non-prescription dietary supplements, such as diosmin,[79] while one other – Vasculera (Diosmiplex) – is a prescription medical food intended for treating venous disorders.[81][82] Adverse effects of polyphenol intake range from mild (e.g., gastrointestinal tract symptoms)[2] to severe (e.g., hemolytic anemia or hepatotoxicity).[84] Polyphenols, particularly in beverages that contain them in high concentrations (tea, coffee, etc), inhibit the absorption of non-haem iron when consumed together in a single meal.[2][83] The European Food Safety Authority established upper limits for some polyphenol-containing supplements and additives, such as green tea extract or curcumin.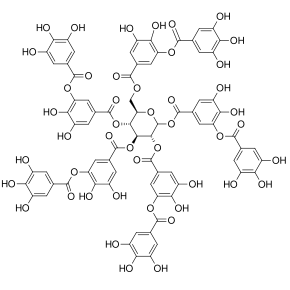





tannic acidphenylpropanoidgallic acidphenolsphenolic acidsflavonoidsellagitannintanning garmentsCurcuminturmericAncient GreekbenzenoidphenylhydroxylEllagic acidhydroxyl groupsaromatic ringscatechinhesperetincyanidindaidzeinsoybeanproanthocyanidinsquercetincaffeic acidLignansphenylalaninetanningwater-solublegalloylRaspberry ellagitannintanninshikimatepolyketideblack teatheaflavin-3-gallatetheaflavinreactiveoxidationantioxidantsin vitromacromoleculesdaltonssenescesesterscondensed tanninsepicatechinisoflavonebenzopyrancarboxylic acidstellimagrandin II1,2,3,4,6-pentagalloyl-glucosephytochemistryextractionstructural elucidationquantificationcoordination complexesMetal-phenolic Networkshot waterLiquid–liquid extractioncountercurrent chromatographySolid phase extractioncritical carbon dioxideultrafiltrationpreparative chromatographynatural phenolstanninsPhosphomolybdic acidthin layer chromatographyspectroscopyfractionationpaper chromatographyInstrumental chemistryseparationhigh performance liquid chromatographyreversed-phase liquid chromatographymass spectrometrynuclear magnetic resonanceDMACA reagentautofluorescencesuberinplant sciencevolumetric titrationpermanganatestandard curvecolorimetricFolin–Ciocalteu reactionantibodycationthiolsvitamin CTrolox equivalent antioxidant capacityTroloxvitamin Ediphenylpicrylhydrazyloxygen radical absorbance capacityferric reducing ability of plasmalow-density lipoproteinbiosensorsdiode array detectorabsolute valuesstandardsleather tanningIndian subcontinentpomegranatephotographic developersrenewablepolymerizationformaldehydeadhesivespomacesnutrient cyclesarthropodscrustaceansepicuticlesclerotizationpolyphenol oxidasepigmentationarachnidsplant pigmentsphytoalexinspreservationMyriophyllum spicatumallelopathicshikimic acidgallotannins
