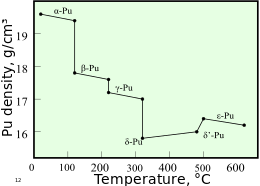
Plutonium
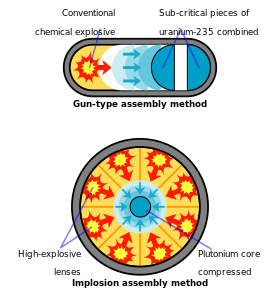
The heavy isotope plutonium-244 has a half-life long enough that extreme trace quantities should have survived primordially (from the Earth's formation) to the present, but so far experiments have not yet been sensitive enough to detect it.The presence of plutonium-240 limits a plutonium sample's usability for weapons or its quality as reactor fuel, and the percentage of plutonium-240 determines its grade (weapons-grade, fuel-grade, or reactor-grade).[17] Plutonium in the δ (delta) form normally exists in the 310 °C to 452 °C range but is stable at room temperature when alloyed with a small percentage of gallium, aluminium, or cerium, enhancing workability and allowing it to be welded.[citation needed] The ε phase, the highest temperature solid allotrope, exhibits anomalously high atomic self-diffusion compared to other elements.[15][43] Powders of plutonium, its hydrides and certain oxides like Pu2O3 are pyrophoric, meaning they can ignite spontaneously at ambient temperature and are therefore handled in an inert, dry atmosphere of nitrogen or argon.[56] These trace amounts of 239Pu originate in the following fashion: on rare occasions, 238U undergoes spontaneous fission, and in the process, the nucleus emits one or two free neutrons with some kinetic energy.[59] Due to its relatively long half-life of about 80 million years, it was suggested that plutonium-244 occurs naturally as a primordial nuclide, but early reports of its detection could not be confirmed.[63] The former presence of 244Pu in the early Solar System has been confirmed, since it manifests itself today as an excess of its daughters, either 232Th (from the alpha decay pathway) or xenon isotopes (from its spontaneous fission).[73] A paper documenting the discovery was prepared by the team and sent to the journal Physical Review in March 1941,[42] but publication was delayed until a year after the end of World War II due to security concerns.[75] At the Cavendish Laboratory in Cambridge, Egon Bretscher and Norman Feather realized that a slow neutron reactor fuelled with uranium would theoretically produce substantial amounts of plutonium-239 as a by-product.[82][note 4] On December 2, 1942, on a racket court under the west grandstand at the University of Chicago's Stagg Field, researchers headed by Enrico Fermi achieved the first self-sustaining chain reaction in a graphite and uranium pile known as CP-1.Using theoretical information garnered from the operation of CP-1, DuPont constructed an air-cooled experimental production reactor, known as X-10, and a pilot chemical separation facility at Oak Ridge.The separation facility, using methods developed by Glenn T. Seaborg and a team of researchers at the Met Lab, removed plutonium from uranium irradiated in the X-10 reactor.The interior had an eerie quality as operators behind seven feet of concrete shielding manipulated remote control equipment by looking through television monitors and periscopes from an upper gallery.[89] The original gun-type plutonium weapon, code-named "Thin Man", had to be abandoned as a result—the increased number of spontaneous neutrons meant that nuclear pre-detonation (fizzle) was likely.[93] By the end of January 1945, the highly purified plutonium underwent further concentration in the completed chemical isolation building, where remaining impurities were removed successfully.[83] According to Kate Brown, the plutonium production plants at Hanford and Mayak in Russia, over a period of four decades, "both released more than 200 million curies of radioactive isotopes into the surrounding environment—twice the amount expelled in the Chernobyl disaster in each instance".[94] Most of this radioactive contamination over the years were part of normal operations, but unforeseen accidents did occur and plant management kept this secret, as the pollution continued unabated.Inside the safe were various items, including a large glass bottle containing a whitish slurry which was subsequently identified as the oldest sample of weapons-grade plutonium known to exist.[107] As noted by Carl Johnson in Ambio, "Exposures of a large population in the Denver area to plutonium and other radionuclides in the exhaust plumes from the plant date back to 1953.[110] On March 5, 2009, Energy Secretary Steven Chu told a Senate hearing "the Yucca Mountain site no longer was viewed as an option for storing reactor waste".[115] For human subjects, this involved injecting solutions typically containing 5 micrograms (μg) of plutonium into hospital patients thought to be either terminally ill, or to have a life expectancy of less than ten years either due to age or chronic disease.[120] The government covered up most of these actions until 1993, when President Bill Clinton ordered a change of policy and federal agencies then made available relevant records.If fast neutron reactors are not available (the normal case), excess plutonium is usually discarded, and forms one of the longest-lived components of nuclear waste.The desire to consume this plutonium and other transuranic fuels and reduce the radiotoxicity of the waste is the usual reason nuclear engineers give to make fast neutron reactors.A dedicated reactor operating on very low burnup (hence minimal exposure of newly formed plutonium-239 to additional neutrons which causes it to be transformed to heavier isotopes of plutonium) is generally required to produce material suitable for use in efficient nuclear weapons.[144][145] Donald Mastick accidentally swallowed a small amount of plutonium(III) chloride, which was detectable for the next thirty years of his life, but appeared to suffer no ill effects.[149] A commonly cited quote by Ralph Nader states that a pound of plutonium dust spread into the atmosphere would be enough to kill 8 billion people.[160] In December 1958, during a process of purifying plutonium at Los Alamos, a critical mass formed in a mixing vessel, which killed chemical operator Cecil Kelley.A typical transport consists of one truck carrying one protected shipping container, holding a number of packages with a total weight varying from 80 to 200 kg of plutonium oxide.











Plutonium (disambiguation)PoloniumAllotropesAllotropes of plutoniumMass numberperiodic tableHydrogenHeliumLithiumBerylliumCarbonNitrogenOxygenFluorineSodiumMagnesiumAluminiumSiliconPhosphorusSulfurChlorinePotassiumCalciumScandiumTitaniumVanadiumChromiumManganeseCobaltNickelCopperGalliumGermaniumArsenicSeleniumBromineKryptonRubidiumStrontiumYttriumZirconiumNiobiumMolybdenumTechnetiumRutheniumRhodiumPalladiumSilverCadmiumIndiumAntimonyTelluriumIodineCaesiumBariumLanthanumCeriumPraseodymiumNeodymiumPromethiumSamariumEuropiumGadoliniumTerbiumDysprosiumHolmiumErbiumThuliumYtterbiumLutetiumHafniumTantalumTungstenRheniumOsmiumIridiumPlatinumMercury (element)ThalliumBismuthAstatineFranciumRadiumActiniumThoriumProtactiniumUraniumNeptuniumAmericiumCuriumBerkeliumCaliforniumEinsteiniumFermiumMendeleviumNobeliumLawrenciumRutherfordiumDubniumSeaborgiumBohriumHassiumMeitneriumDarmstadtiumRoentgeniumCoperniciumNihoniumFleroviumMoscoviumLivermoriumTennessineOganessonAtomic numberf-block groupsPeriodperiod 7Electron configurationMelting pointBoiling pointDensityHeat of fusionkJ/molHeat of vaporizationMolar heat capacityVapor pressureOxidation states