Molybdenum
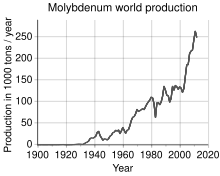
[13] Molybdenum does not occur naturally as a free metal on Earth; in its minerals, it is found only in oxidized states.Heating molybdenum-bearing minerals under oxygen and water affords molybdate ion MoO2−4, which forms quite soluble salts.[17] In its pure form, molybdenum is a silvery-grey metal with a Mohs hardness of 5.5 and a standard atomic weight of 95.95 g/mol.It does not visibly react with oxygen or water at room temperature, but is attacked by halogens and hydrogen peroxide.[40] By 1778 Swedish chemist Carl Wilhelm Scheele stated firmly that molybdena was (indeed) neither galena nor graphite.[41][42] Instead, Scheele correctly proposed that molybdena was an ore of a distinct new element, named molybdenum for the mineral in which it resided, and from which it might be isolated.In 1906, William D. Coolidge filed a patent for rendering molybdenum ductile, leading to applications as a heating element for high-temperature furnaces and as a support for tungsten-filament light bulbs; oxide formation and degradation require that molybdenum be physically sealed or held in an inert gas.[48] During World War I, demand for molybdenum spiked; it was used both in armor plating and as a substitute for tungsten in high-speed steels.[50] After the war, demand plummeted until metallurgical advances allowed extensive development of peacetime applications.In World War II, molybdenum again saw strategic importance as a substitute for tungsten in steel alloys.[20][52] The Soviet Luna 24 mission discovered a molybdenum-bearing grain (1 × 0.6 μm) in a pyroxene fragment taken from Mare Crisium on the Moon.[53] The comparative rarity of molybdenum in the Earth's crust is offset by its concentration in a number of water-insoluble ores, often combined with sulfur in the same way as copper, with which it is often found.[12] The world's production of molybdenum was 250,000 tonnes in 2011, the largest producers being China (94,000 t), the United States (64,000 t), Chile (38,000 t), Peru (18,000 t) and Mexico (12,000 t).[61] Molybdenum can withstand extreme temperatures without significantly expanding or softening, making it useful in environments of intense heat, including military armor, aircraft parts, electrical contacts, industrial motors, and supports for filaments in light bulbs.[62] TZM (Mo (~99%), Ti (~0.5%), Zr (~0.08%) and some C) is a corrosion-resisting molybdenum superalloy that resists molten fluoride salts at temperatures above 1,300 °C (2,370 °F).[66] It is used as the valve body of torpedo engines, rocket nozzles and gas pipelines, where it can withstand extreme thermal and mechanical stresses.[70] Molybdenum, despite its low concentration in the environment, is a critically important element for Earth's biosphere due to its presence in the most common nitrogenases.[88] In terms of function, molybdoenzymes catalyze the oxidation and sometimes reduction of certain small molecules in the process of regulating nitrogen, sulfur, and carbon.[91][92] Nitrogenases catalyze the production of ammonia from atmospheric nitrogen: The biosynthesis of the FeMoco active site is highly complex.[100] Although human toxicity data is unavailable, animal studies have shown that chronic ingestion of more than 10 mg/day of molybdenum can cause diarrhea, growth retardation, infertility, low birth weight, and gout; it can also affect the lungs, kidneys, and liver.[106] Molybdenum deficiency has also been reported as a consequence of non-molybdenum supplemented total parenteral nutrition (complete intravenous feeding) for long periods of time.Ruminants that consume high levels of molybdenum suffer from diarrhea, stunted growth, anemia, and achromotrichia (loss of fur pigment).It has also been found to have an inhibitory effect on angiogenesis, potentially by inhibiting the membrane translocation process that is dependent on copper ions.[114] This is a promising avenue for investigation of treatments for cancer, age-related macular degeneration, and other diseases that involve a pathologic proliferation of blood vessels.Molybdenum targets are used in mammography because they produce X-rays in the energy range of 17-20 keV, which is optimal for imaging soft tissues like the breast.[119] This energy range also minimizes radiation dose while maximizing image quality, making molybdenum targets particularly suitable for breast cancer screening.As for safety, the NAM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient.[123] For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV).Other significant dietary sources include green beans, eggs, sunflower seeds, wheat flour, lentils, cucumbers, and cereal grain.



Standard atomic weightperiodic tableHydrogenHeliumLithiumBerylliumCarbonNitrogenOxygenFluorineSodiumMagnesiumAluminiumSiliconPhosphorusSulfurChlorinePotassiumCalciumScandiumTitaniumVanadiumChromiumManganeseCobaltNickelCopperGalliumGermaniumArsenicSeleniumBromineKryptonRubidiumStrontiumYttriumZirconiumNiobiumTechnetiumRutheniumRhodiumPalladiumSilverCadmiumIndiumAntimonyTelluriumIodineCaesiumBariumLanthanumCeriumPraseodymiumNeodymiumPromethiumSamariumEuropiumGadoliniumTerbiumDysprosiumHolmiumErbiumThuliumYtterbiumLutetiumHafniumTantalumTungstenRheniumOsmiumIridiumPlatinumMercury (element)ThalliumBismuthPoloniumAstatineFranciumRadiumActiniumThoriumProtactiniumUraniumNeptuniumPlutoniumAmericiumCuriumBerkeliumCaliforniumEinsteiniumFermiumMendeleviumNobeliumLawrenciumRutherfordiumDubniumSeaborgiumBohriumHassiumMeitneriumDarmstadtiumRoentgeniumCoperniciumNihoniumFleroviumMoscoviumLivermoriumTennessineOganessonAtomic numbergroup 6Periodperiod 5Electron configurationMelting pointBoiling pointDensityHeat of fusionkJ/molHeat of vaporizationMolar heat capacityVapor pressureOxidation statesElectronegativityIonization energiesAtomic radius