Titanium
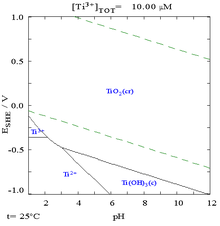
Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in sea water, aqua regia, and chlorine.Titanium was discovered in Cornwall, Great Britain, by William Gregor in 1791 and was named by Martin Heinrich Klaproth after the Titans of Greek mythology.The element occurs within a number of minerals, principally rutile and ilmenite, which are widely distributed in the Earth's crust and lithosphere; it is found in almost all living things, as well as bodies of water, rocks, and soils.The resulting titanium alloys are strong, lightweight, and versatile, with applications including aerospace (jet engines, missiles, and spacecraft), military, industrial processes (chemicals and petrochemicals, desalination plants, pulp, and paper), automotive, agriculture (farming), sporting goods, jewelry, and consumer electronics.[31] Significant titanium-bearing ilmenite deposits exist in Australia, Canada, China, India, Mozambique, New Zealand, Norway, Sierra Leone, South Africa, and Ukraine.The identity of titanium species in aqueous solution remains unknown because of its low solubility and the lack of sensitive spectroscopic methods, although only the 4+ oxidation state is stable in air.Ti3O5, described as a Ti(IV)-Ti(III) species, is a purple semiconductor produced by reduction of TiO2 with hydrogen at high temperatures,[41] and is used industrially when surfaces need to be vapor-coated with titanium dioxide: it evaporates as pure TiO, whereas TiO2 evaporates as a mixture of oxides and deposits coatings with variable refractive index.[43] The alkoxides of titanium(IV), prepared by treating TiCl4 with alcohols, are colorless compounds that convert to the dioxide on reaction with water.[45] Titanium nitride (TiN) is a refractory solid exhibiting extreme hardness, thermal/electrical conductivity, and a high melting point.[46] TiN has a hardness equivalent to sapphire and carborundum (9.0 on the Mohs scale),[47] and is often used to coat cutting tools, such as drill bits.Despite these advantages the first candidate compounds failed clinical trials due to insufficient efficacy to toxicity ratios and formulation complications.[30] Realizing that the unidentified oxide contained a metal that did not match any known element, in 1791 Gregor reported his findings in both German and French science journals: Crell's Annalen and Observations et Mémoires sur la Physique.[13] The oxide was independently rediscovered in 1795 by Prussian chemist Martin Heinrich Klaproth in rutile from Boinik (the German name of Bajmócska), a village in Hungary (now Bojničky in Slovakia).[12][13] Titanium of very high purity was made in small quantities when Anton Eduard van Arkel and Jan Hendrik de Boer discovered the iodide process in 1925, by reacting with iodine and decomposing the formed vapors over a hot filament to pure metal.[73] The process involves reducing titanium tetrachloride (TiCl4) with sodium (Na) in a batch reactor with an inert atmosphere at a temperature of 1,000 °C."[79] A 2023 review "discusses the electrochemical principles involved in the recovery of metals from aqueous solutions and fused salt electrolytes", with particular attention paid to titanium.[24] Contamination causes a variety of conditions, such as embrittlement, which reduce the integrity of the assembly welds and lead to joint failure.[87] Commercially pure flat product (sheet, plate) can be formed readily, but processing must take into account of the tendency of the metal to springback.[88][89] Exposure to the oxygen in air at the elevated temperatures used in forging results in formation of a brittle oxygen-rich metallic surface layer called "alpha case" that worsens the fatigue properties, so it must be removed by milling, etching, or electrochemical treatment.[11] Titanium is often alloyed with aluminium (to refine grain size), vanadium, copper (to harden), iron, manganese, molybdenum, and other metals.[96] Titanium mill products (sheet, plate, bar, wire, forgings, castings) find application in industrial, aerospace, recreational, and emerging markets.[12][13] For these applications, titanium is alloyed with aluminium, zirconium, nickel,[100] vanadium, and other elements to manufacture a variety of components including critical structural parts, landing gear, firewalls, exhaust ducts (helicopters), and hydraulic systems.The pulp and paper industry uses titanium in process equipment exposed to corrosive media, such as sodium hypochlorite or wet chlorine gas (in the bleachery).Titanium tetrachloride is also used to iridize glass and, because it fumes strongly in moist air, it is used to make smoke screens.[19] Titanium metal is used in automotive applications, particularly in automobile and motorcycle racing where low weight and high strength and rigidity are critical.[114] Titanium alloys are used in spectacle frames that are rather expensive but highly durable, long lasting, light weight, and cause no skin allergies.[116][117] The Guggenheim Museum Bilbao and the Cerritos Millennium Library were the first buildings in Europe and North America, respectively, to be sheathed in titanium panels.[123] Titanium may be anodized to vary the thickness of the surface oxide layer, causing optical interference fringes and a variety of bright colors.[126] The Gold Coast Titans, an Australian rugby league team, award a medal of pure titanium to their player of the year.[132] Titanium is used for the surgical instruments used in image-guided surgery, as well as wheelchairs, crutches, and any other products where high strength and low weight are desirable.














Titanium (disambiguation)Standard atomic weightperiodic tableHydrogenHeliumLithiumBerylliumCarbonNitrogenOxygenFluorineSodiumMagnesiumAluminiumSiliconPhosphorusSulfurChlorinePotassiumCalciumScandiumVanadiumChromiumManganeseCobaltNickelCopperGalliumGermaniumArsenicSeleniumBromineKryptonRubidiumStrontiumYttriumZirconiumNiobiumMolybdenumTechnetiumRutheniumRhodiumPalladiumSilverCadmiumIndiumAntimonyTelluriumIodineCaesiumBariumLanthanumCeriumPraseodymiumNeodymiumPromethiumSamariumEuropiumGadoliniumTerbiumDysprosiumHolmiumErbiumThuliumYtterbiumLutetiumHafniumTantalumTungstenRheniumOsmiumIridiumPlatinumMercury (element)ThalliumBismuthPoloniumAstatineFranciumRadiumActiniumThoriumProtactiniumUraniumNeptuniumPlutoniumAmericiumCuriumBerkeliumCaliforniumEinsteiniumFermiumMendeleviumNobeliumLawrenciumRutherfordiumDubniumSeaborgiumBohriumHassiumMeitneriumDarmstadtiumRoentgeniumCoperniciumNihoniumFleroviumMoscoviumLivermoriumTennessineOganessonAtomic numbergroup 4Periodperiod 4Electron configurationMelting pointBoiling pointDensityHeat of fusionkJ/molHeat of vaporizationMolar heat capacityVapor pressureOxidation statesElectronegativityIonization energies
