Isopropyl alcohol
[9] Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins.Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm.It is produced through hydration of propene or hydrogenation of acetone, with modern processes achieving anhydrous alcohol through azeotropic distillation.Despite its utility, isopropyl alcohol poses safety risks due to its flammability and potential for peroxide formation.Its ingestion or absorption leads to toxic effects including central nervous system depression and coma, primarily treated through supportive measures.The process is colloquially called salting out, and causes concentrated isopropyl alcohol to separate into a distinct layer.These processes give primarily isopropyl alcohol rather than 1-propanol, because adding water or sulfuric acid to propene follows Markovnikov's rule.[18] Direct hydration reacts propene and water, either in gas or liquid phase, at high pressures in the presence of solid or supported acidic catalysts.It evaporates quickly and the typically available grades tend to not leave behind oil traces when used as a cleaning fluid unlike some other common solvents.Isopropyl alcohol is commonly used for cleaning eyeglasses, electrical contacts, audio or video tape heads, DVD and other optical disc lenses, bongs,[22] and for removing thermal paste from heatsinks on CPUs[23] and other IC packages.[26] Inhaled isopropyl alcohol can be used for treating nausea in some settings by placing a disinfecting pad under the nose.Isopropyl alcohol can also be used similarly to ether as a solvent[28] or as an anesthetic by inhaling the fumes or orally.However, it was soon discontinued, as many complications arose, including respiratory irritation, internal bleeding, and visual and hearing problems.In significant quantities, water is a problem in fuel tanks, as it separates from gasoline and can freeze in the supply lines at low temperatures.[35] Unlike methanol or ethylene glycol poisoning, the metabolites of isopropyl alcohol are considerably less toxic, and treatment is largely supportive.Furthermore, there is no indication for the use of fomepizole, an alcohol dehydrogenase inhibitor, unless co-ingestion with methanol or ethylene glycol is suspected.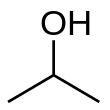




Preferred IUPAC nameRubbing alcoholCAS NumberBeilstein ReferenceChEMBLChemSpiderECHA InfoCardGmelin ReferencePubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterMiscibleSolubilitybenzenechloroformethanoldiethyl etherglycerolacetoneAcidityMagnetic susceptibilityRefractive indexViscosityATC codeD08AX05Occupational safety and healthGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointAutoignitiontemperatureExplosive limitsThreshold limit valueSafety data sheetalcohols1-Propanol2-butanolIsopropyl alcohol (data page)standard statecolorlessflammableorganic compoundpolar moleculeethyl cellulosepolyvinyl butyralalkaloidsresinssodium chloridesalting outazeotropeviscousultravioletisopropylhydroxylalcoholstructural isomerpropan-1-olethyl methyl etherAlexander William Williamsoncorditepropenehydrogenationazeotropic distillationhand sanitizerantisepticsdisinfectantsdetergentsperoxidecentral nervous system depressionorganicmethanolaqueous solutionsabsorbancevisibleoxidizedketonechromic aciddehydrogenationcatalysthydrideMeerwein-Ponndorf-Verley reductiontransfer hydrogenation2-bromopropanephosphorus tribromidesulfuric acidmetalspotassiumalkoxidestitanium tetrachloridetitanium isopropoxidealuminiummercuryaluminium isopropoxideStandard Oillow explosivehydration reactiondistillationanhydrousdiisopropyl ethercyclohexanediabetic ketoacidosishydrationMarkovnikov's rulehydrolysisHeteropoly acidcatalystspropylenecumene processbyproductphenolRaney nickelsupportedcloud chamberssupersaturatedcondensedparticles