Solubility
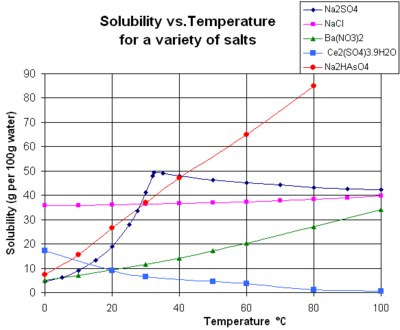
Gases are always miscible in all proportions, except in very extreme situations,[3] and a solid or liquid can be "dissolved" in a gas only by passing into the gaseous state first.The solubility mainly depends on the composition of solute and solvent (including their pH and the presence of other dissolved substances) as well as on temperature and pressure.The dependency can often be explained in terms of interactions between the particles (atoms, molecules, or ions) of the two substances, and of thermodynamic concepts such as enthalpy and entropy.The concept and measure of solubility are extremely important in many sciences besides chemistry, such as geology, biology, physics, and oceanography, as well as in engineering, medicine, agriculture, and even in non-technical activities like painting, cleaning, cooking, and brewing.[7] Moreover, many solids (such as acids and salts) will dissociate in non-trivial ways when dissolved; conversely, the solvent may form coordination complexes with the molecules or ions of the solute.For example, the solubility of aragonite and calcite in water are expected to differ, even though they are both polymorphs of calcium carbonate and have the same chemical formula.[clarification needed] The solubility of one substance in another is determined by the balance of intermolecular forces between the solvent and solute, and the entropy change that accompanies the solvation.Solubility may also strongly depend on the presence of other species dissolved in the solvent, for example, complex-forming anions (ligands) in liquids.Solubility will also depend on the excess or deficiency of a common ion in the solution[clarification needed], a phenomenon known as the common-ion effect.[citation needed] For example, they provide the driving force for precipitate aging (the crystal size spontaneously increasing with time).[12] In liquid water at high temperatures, (e.g. that approaching the critical temperature), the solubility of ionic solutes tends to decrease due to the change of properties and structure of liquid water; the lower dielectric constant results in a less polar solvent and in a change of hydration energy affecting the ΔG of the dissolution reaction.[12] The chart shows solubility curves for some typical solid inorganic salts in liquid water (temperature is in degrees Celsius, i.e. kelvins minus 273.15).[14] Many salts behave like barium nitrate and disodium hydrogen arsenate, and show a large increase in solubility with temperature (ΔH > 0).[16] For condensed phases (solids and liquids), the pressure dependence of solubility is typically weak and usually neglected in practice.The decrease of solubility of carbon dioxide in seawater when temperature increases is also an important retroaction factor (positive feedback) exacerbating past and future climate changes as observed in ice cores from the Vostok site in Antarctica.When a deglaciation period is initiated, the progressive warming of the oceans releases CO2 into the atmosphere because of its lower solubility in warmer sea water.In turn, higher levels of CO2 in the atmosphere increase the greenhouse effect and carbon dioxide acts as an amplifier of the general warming.[20] In even more simple terms a simple ionic compound (with positive and negative ions) such as sodium chloride (common salt) is easily soluble in a highly polar solvent (with some separation of positive (δ+) and negative (δ-) charges in the covalent molecule) such as water, as thus the sea is salty as it accumulates dissolved salts since early geological ages.Chemists often exploit differences in solubilities to separate and purify compounds from reaction mixtures, using the technique of liquid-liquid extraction.In some cases, solubility equilibria can take a long time to establish (hours, days, months, or many years; depending on the nature of the solute and other factors).Solubility is of fundamental importance in a large number of scientific disciplines and practical applications, ranging from ore processing and nuclear reprocessing to the use of medicines, and the transport of pollutants.For example, indigo is described as "insoluble in water, alcohol, or ether but soluble in chloroform, nitrobenzene, or concentrated sulfuric acid".The other reaction products, including the magnesium bromide, will remain in the aqueous layer, clearly showing that separation based on solubility is achieved.This equilibrium constant depends on the type of salt (AgCl vs. NaCl, for example), temperature, and the common ion effect.This term is often used in the field of metallurgy to refer to the extent that an alloying element will dissolve into the base metal without forming a separate phase.[24] Besides these, the ZnSb compound with Sn as a solute can separate out into other combinations of phases after the solubility limit is reached depending on the initial chemical composition during synthesis.Solubility is a property of interest in many aspects of science, including but not limited to: environmental predictions, biochemistry, pharmacy, drug-design, agrochemical design, and protein ligand binding.The ability to accurately predict a molecule's solubility represents potentially large financial savings in many chemical product development processes, such as pharmaceuticals.[31] In the pharmaceutical industry, solubility predictions form part of the early stage lead optimisation process of drug candidates.A method founded in physical theory, capable of achieving similar levels of accuracy at an sensible cost, would be a powerful tool scientifically and industrially.




solvable groupsolution (disambiguation)ammonium sulfatechemistrysubstancesolutesolutionsolventconcentrationsolubility equilibriummiscibleliquidmoleculesthermodynamicenthalpyentropysupersaturated solutionmetastablenucleationchemical reactioncalcium hydroxidehydrochloric acidrate of solutiongeologybiologyphysicsoceanographyengineeringmedicineagriculturepaintingcleaningcookingbrewingreagentscolloidal suspensionsvolumeamount in moleshandbooksmillilitresdecilitrekilogramkilogramsmolalitytitrationmolaritymole fractionmass fractiondimensionlesspercentagesdissociatecoordination complexesethanoltitanium dioxideU.S. Pharmacopoeiacalcium nitratecalcium chloridesodium oxalatecalcium sulfatedicalcium phosphatebarium sulfatedissolutionprecipitationsolidssolvolysisoxidesaqueousphasesaragonitecalcitepolymorphscalcium carbonatechemical formulaintermolecular forcescomplex-formingligandscommon-ion effectionic strengthredox bufferspecific surface areaprecipitate agingendothermicexothermicvan 't Hoff equationequilibrium constantcritical temperaturedielectric constantpolar solventGaseousCelsiuskelvinsbarium nitratedisodium hydrogen arsenatesodium chloridegypsumcerium(III) sulfateportlanditeLe Chatelier's principlesodium sulfatehydratemirabilitewater of crystallizationanhydrousthenarditeGibbs free energyorganic compoundsrecrystallizationcyclodextrinsideal solutionpartial molar volumeuniversal gas constantHenry's lawpartial pressureRaoult's lawBunsen solubility coefficientbubblesYoung–Laplace equationSieverts' lawcarbon dioxideseawatercarbonateclimate changesAntarcticageological timeMilankovich cyclessolar irradiance