Dinitrogen tetroxide
Dinitrogen tetroxide is a powerful oxidizer that is hypergolic (spontaneously reacts) upon contact with various forms of hydrazine, which has made the pair a common bipropellant for rockets.[9] Nitrogen tetroxide is made by the catalytic oxidation of ammonia (the Ostwald process): steam is used as a diluent to reduce the combustion temperature.Nitrogen tetroxide is used as an oxidizing agent in one of the most important rocket propellant systems because it can be stored as a liquid at room temperature.[14] Paulet would soon be approached by Nazi Germany to help develop rocket technology, though he refused to assist and never shared the formula for his propellant.[17] On 24 July 1975, NTO poisoning affected three U.S. astronauts on the final descent to Earth after the Apollo-Soyuz Test Project flight.[20] "Cool" dinitrogen tetroxide is compressed and heated, causing it to dissociate into nitrogen dioxide at half the molecular weight.[21] The high molecular weight and smaller volumetric expansion ratio of nitrogen dioxide compared to steam allows the turbines to be more compact.When exposed to oxygen, NO is converted back into nitrogen dioxide: The resulting NO2 and N2O4 can be returned to the cycle to give the mixture of nitrous and nitric acids again.This branch of chemistry was developed by Cliff Addison and Norman Logan at the University of Nottingham in the UK during the 1960s and 1970s when highly efficient desiccants and dry boxes started to become available.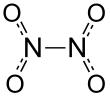



Nitrogen dioxideIUPAC nameCAS NumberChemSpiderECHA InfoCardEC NumberGmelin ReferencePubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterVapor pressureMagnetic susceptibilityRefractive indexMolecular shapeStd molarentropyStd enthalpy offormationGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointSafety data sheetnitrogenoxidesNitrous oxideNitric oxideDinitrogen trioxideDinitrogen pentoxidePhosphorus tetroxideArsenic tetroxideAntimony tetroxidestandard statechemical compoundreagentequilibrium mixtureoxidizerhypergolichydrazinebipropellantnitro groupsdiamagneticcatalyticoxidationammoniaOstwald processdiluentnitric acidPedro PauletPeruvianpolymathpetroleum benzinespark plugVerein für Raumschiffahrt (VfR)Nazi GermanyS-StoffUnited Stateshypergolic propellantrocket fuelTitan family of rocketslaunch vehiclesGeminiApolloSpace ShuttleProton rocketstress-corrosion crackingmixed oxides of nitrogenastronautsApollo-Soyuz Test Projectattitude controlpneumoniapulmonary edemaBrayton cyclesnitrous aciddisproportionatesmolecular autoionizationnitrosoniumtransition metal nitrate complexeshydrated metal ioncopper nitrateCliff AddisonUniversity of Nottinghamdry boxesalkenesnitro compoundsnitrite estersnitroso compoundsnitrate estersEl ComercioBBC NewsCambridge UniversityWayback MachinePoliakoff, MartynThe Periodic Table of Videos−CONR2–NO2NO−2NO−3(HON)2N2O2−2H2NNO2ONOO−HO2NO2O2NOO−NO3−4H4N2O4N2O2−3Oxidation statesacidicBoron suboxideCarbon suboxideChlorine perchlorateChloryl perchlorateCobalt(II,III) oxideDichlorine pentoxideIron(II,III) oxideLead(II,IV) oxideManganese(II,III) oxide