Copper(I) oxide
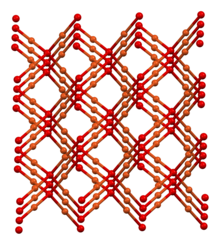
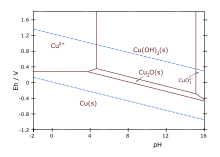
Alternatively, it may be prepared via the reduction of copper(II) acetate with hydrazine:[4] Aqueous cuprous chloride solutions react with base to give the same material.These sugars reduce an alkaline solution of a copper(II) salt, giving a bright red precipitate of Cu2O.It forms on silver-plated copper parts exposed to moisture when the silver layer is porous or damaged.The structure thus resembles in some sense the main polymorphs of SiO2, but cuprous oxide's lattices interpenetrate.Another unusual feature of the ground state excitons is that all primary scattering mechanisms are known quantitatively.[11] In December 2021, Toshiba disclosed a transparent cuprous oxide (Cu2O) thin-film solar cell.



Unit cellCrystal packingIUPAC nameCupriteCAS NumberChemSpiderECHA InfoCardEC NumberPubChemRTECS numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilityBand gapMagnetic susceptibilityCrystal structureSpace groupLattice constantStd molarentropyStd enthalpy offormationGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Safety data sheetanionsCopper(I) sulfideCopper(II) sulfideCopper(I) selenidecationsCopper(II) oxideSilver(I) oxideNickel(II) oxideZinc oxidestandard stateinorganic compoundoxidescoppermineralantifoulingsemiconductoroxidationsulfur dioxidecopper(II) acetatehydrazineAqueouscuprous chloridePourbaix diagramFehling's testBenedict's testsugarsalkalineprecipitatesilvercorrosionred plaguediamagneticcuprous hydroxideammoniacomplexoxidizedHydrochloric acidchloride complexSulfuric acidnitric acidcopper(II) sulfatecopper(II) nitratetetrahedralpoint grouppigmentfungicideRectifier diodessilicondiodesexcitonphotonphononpolaritonsgroup velocityground stateabsorptionlinewidthtemperatureabsorption coefficientKramers–Kronig relationsToshibasolar cellenergy conversion efficiencyelectric vehiclesparamelaconiteCopper(III) oxideNational Institute for Occupational Safety and HealthBibcodeAcademic PressNew York CityScientific AmericanWayback MachineCopper compoundsCu2Cr2O5C6H5CuCu3H4O8S2Cu(BF4)2Cu(CH3COO)2Cu(CF3COO)2Cu(C3H5O3)2Cu2CO3(OH)2KCuCl3K2CuCl4Cu(ClO3)2Cu(ClO4)2Cu(NO3)2Cu3(PO4)2Cu3(BO3)2Cu(N3)2CuC2O4Cu(OH)2Cu(SCN)2Cu3(AsO4)2Cu(C11H23COO)2