Nuclear magnetic resonance spectroscopy
This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field.As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule.Besides identification, NMR spectroscopy provides detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.NMR has largely replaced traditional wet chemistry tests such as color reagents or typical chromatography for identification.Less expensive machines using permanent magnets and lower resolution are also available, which still give sufficient performance for certain applications such as reaction monitoring and quick checking of samples.Low-resolution NMR produces broader peaks, which can easily overlap one another, causing issues in resolving complex structures.Atoms with an odd sum of protons and neutrons exhibit half-integer values for the nuclear spin quantum number (I = 1/2, 3/2, 5/2, and so on).[12] The resonant frequency, energy of the radiation absorbed, and the intensity of the signal are proportional to the strength of the magnetic field.[citation needed] An NMR spectrometer typically consists of a spinning sample-holder inside a very strong magnet, a radio-frequency emitter, and a receiver with a probe (an antenna assembly) that goes inside the magnet to surround the sample, optionally gradient coils for diffusion measurements, and electronics to control the system.High-resolution NMR spectrometers use shims to adjust the homogeneity of the magnetic field to parts per billion (ppb) in a volume of a few cubic centimeters.[18][19] Upon excitation of the sample with a radio frequency (60–1000 MHz) pulse, a nuclear magnetic resonance response – a free induction decay (FID) – is obtained.If the second excitation pulse is sent prematurely before the relaxation is complete, the average magnetization vector has not decayed to ground state, which affects the strength of the signal in an unpredictable manner.The energy difference ΔE between nuclear spin states is proportional to the magnetic field (Zeeman effect).For diamagnetic organic compounds, assignments of 1H and 13C NMR spectra are extremely sophisticated because of the large databases and easy computational tools.The chemical shifts for many heavier nuclei are more strongly influenced by other factors, including excited states ("paramagnetic" contribution to shielding tensor).This coupling arises from the interaction of different spin states through the chemical bonds of a molecule and results in the splitting of NMR signals.If the shift separation decreases (or the coupling strength increases), the multiplet intensity patterns are first distorted, and then become more complex and less easily analyzed (especially if more than two spins are involved).Such effects are more common in NMR spectra of aromatic and other non-flexible systems, while conformational averaging about C−C bonds in flexible molecules tends to equalize the couplings between protons on adjacent carbons, reducing problems with magnetic inequivalence.The first two-dimensional experiment, COSY, was proposed by Jean Jeener, a professor at Université Libre de Bruxelles, in 1971.[27] A variety of physical circumstances do not allow molecules to be studied in solution, and at the same time not by other spectroscopic techniques to an atomic level, either.In solid-phase media, such as crystals, microcrystalline powders, gels, anisotropic solutions, etc., it is in particular the dipolar coupling and chemical shift anisotropy that become dominant to the behaviour of the nuclear spin systems.[citation needed] Applications in which solid-state NMR effects occur are often related to structure investigations on membrane proteins, protein fibrils or all kinds of polymers, and chemical analysis in inorganic chemistry, but also include "exotic" applications like the plant leaves and fuel cells.For example, Rahmani et al. studied the effect of pressure and temperature on the bicellar structures' self-assembly using deuterium NMR spectroscopy.A common goal of these investigations is to obtain high resolution 3-dimensional structures of the protein, similar to what can be achieved by X-ray crystallography.In contrast to X-ray crystallography, NMR spectroscopy is usually limited to proteins smaller than 35 kDa, although larger structures have been solved.Because of the much higher number of atoms present in a protein molecule in comparison with a small organic compound, the basic 1D spectra become crowded with overlapping signals to an extent where direct spectral analysis becomes untenable.The most important method used for structure determination of proteins utilizes NOE experiments to measure distances between atoms within the molecule.[clarification needed] High-pressure NMR spectroscopy has been widely used for a variety of applications, mainly related to the characterization of the structure of protein molecules.[39][40] However, in recent years, software and design solutions have been proposed to characterize the chemical and spatial structures of small molecules in a supercritical fluid environment,[41] using state parameters as a driving force for such changes.


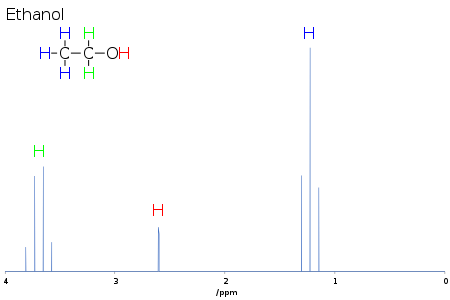


spectroscopicatomic nucleinuclear spinsradio frequencyisotopicfunctional groupsorganic compoundsproteinsprotoncarbon-13 NMRsmall moleculeswet chemistrycolor reagentsmass spectrometrysolid-state NMRtwo-dimensional NMRnuclear Overhauser effectrelaxationliquid-heliumsuperconductingpopulation differencebenchtop nuclear magnetic resonance spectrometersEarth magnetic fieldIsidor Isaac RabiHarvard UniversityStanford UniversityEdward Mills PurcellFelix BlochNobel Prize in Physicselectromagnetic radiationisotopehydrogenprotonsnuclear magnetic momentsNMR tubediffusion constantshydrocarbonsdeuterated solventsdeuteriumdeuterochloroformhydrogen bondingProton NMRinternal standardfree induction decayFourier transformsignal-to-noise ratiosine curveChemical shiftZeeman effecttetramethylsilaneexcited statesparamagnetismparamagnetic NMR spectroscopyJ-couplingspin–spin couplingPascal's trianglecyclicaromaticethanol−OH groupCH3−−CH2−spin quantum numbermentholmethodJohn PopleMagnetic inequivalence2D-NMRCorrelation spectroscopyacronymmoleculeRichard R. Ernstmagic angleNuclear magnetic resonance spectroscopy of proteinsprotein NMRstructural biologyX-ray crystallographyintrinsically unstructured proteinsConformation Activity Relationshipsorders of magnitudeisotopicallyNOE experimentsdistance geometryNuclear magnetic resonance spectroscopy of nucleic acidsNucleic acid NMRnucleic acidsnucleic acid double helices1H or proton NMR13C NMR15N NMR31P NMRcoupling constantsglycosidic bonddihedral anglesKarplus equationcoaxial stackingstem-loopspseudoknotsnucleic acid moleculesNuclear magnetic resonance spectroscopy of carbohydratesCarbohydrate NMRcarbohydratesEarth's field NMRIn vivo magnetic resonance spectroscopyFunctional magnetic resonance spectroscopy of the brainLow field NMRMagnetic Resonance ImagingNMR crystallographyNMR spectra databaseNMR spectroscopy of stereoisomersNuclear quadrupole resonancePulsed field magnetProton-enhanced nuclear induction spectroscopyTriple-resonance nuclear magnetic resonance spectroscopyZero field NMRNuclear magnetic resonance decouplingQuantum mechanics of nuclear magnetic resonance (NMR) spectroscopynuclear spectroscopyMössbauer effectMuon spin spectroscopyPerturbed angular correlationCharles P. SlichterBibcodeChem. Sci.University of CambridgeUniversity of California, Irvine
