Goldschmidt classification
The Goldschmidt classification,[1][2] developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile (sulfide ore-loving or chalcogen-loving), and atmophile (gas-loving) or volatile (the element, or a compound in which it occurs, is liquid or gaseous at ambient surface conditions).Several transition metals, including chromium, molybdenum, iron and manganese, show both lithophile and siderophile characteristics and can be found in both these two layers.The non-metals phosphorus and the halogens were also not known to early chemists, though production of these elements is less difficult than of metallic lithophiles since electrolysis is required only with fluorine.Siderophile elements (from Ancient Greek σίδηρος (sídēros) 'iron') are the transition metals which tend to sink towards the core during planetary differentiation, because they dissolve readily in iron either as solid solutions or in the molten state.The chalcophile elements (from Ancient Greek χαλκός (khalkós) 'copper, brass, bronze', also 'ore') include Ag, As, Bi, Cd, Cu, Ga, Ge, Hg, In, Pb, S, Sb, Se, Sn, Te, Tl and Zn.[6] Chalcophile elements are those that remain on or close to the surface because they combine readily with sulfur and some other chalcogens other than oxygen, forming compounds which did not sink along with iron towards the Earth's core.Chalcophile elements are those metals and heavier nonmetals that have a low affinity for oxygen and prefer to bond with sulfur as highly insoluble sulfides.However, because they formed volatile hydrides in the accreting protosolar nebula when the controlling redox reaction was the oxidation or reduction of hydrogen, the less metallic chalcophile elements are strongly depleted on Earth as a whole relative to cosmic abundances.These greatest enrichments occur in high plateaus like the Tibetan Plateau and the Bolivian Altiplano where large quantities of chalcophile elements have been uplifted through plate tectonics.(In fact they, along with neon, were all first isolated and described by William Ramsay and Morris Travers and assistants, who gave them names with Ancient Greek derivations of 'hidden', 'stranger', and 'new', respectively.)Argon is the exception among the noble gases: it is the third-most abundant component of Earth's present-day atmosphere after nitrogen and oxygen, comprising approx.Trace radioactive elements (namely Tc, Pm, Po, At, Rn, Fr, Ra, Ac, Pa, Np, Pu) are also treated as synthetic.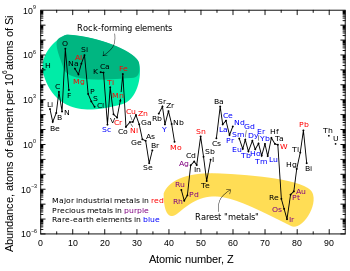
Victor Goldschmidtgeochemical classificationchemical elementssulfide orechalcogenperiodic tablePeriodAncient GreekEarth's corelanthanidesf-blocksd-blocktitaniumzirconiumvanadiumelectron configurationcovalent bondspi bondingsilicaplanetary differentiationalkali metalsseawaterarid regionscontinental shieldsmetallic hydridesEarth's formationrubidiumstrontiumbariumpercent by masselements heavier than ironphosphorushalogenspegmatitesfluorinehydridehydrogen bondschromiummolybdenummanganeseelectrolysismagnesiumaluminiumreducing agentssodiumcalciumchlorineoxidizing agentsodium chlorideSiderophilic bacteriaIron overloadcontinental crustmeteoroidtransition metalssolid solutionsgermaniumniobiumwhen free oxygen did not existrutheniumrhodiumpalladiumrheniumosmiumiridiumplatinumcobaltnickeltungstensilverthermodynamically unstablecarbonsulfurmetallic bondingprecious metalsabundance by massdepositserosionultramafic rockscrustal abundancesEarth's mantlechalcogenssulfidesaccreting protosolar nebularedox reactionseleniumtelluriumhydrogen selenidehydrogen telluridegalliumbauxitealuminum hydroxidereductionTibetan PlateauAltiplanoplate tectonicsmercurynoble gasesvolatile elementsmonatomic gasesnitrogenoxidesdevelopment of free oxygenammoniaoxygencarbon monoxidecarbon dioxidevolcanoesresidence timewater of crystallizationgypsumhydrogen bondingformation of the EarthkryptonWilliam RamsayMorris TraversEarth's present-day atmosphereArgon-40daughterradiogenicSynthetic elementslong-lived parentspoloniumuraniumstandard conditionsnuclear reactorsAbundance of the chemical elements