Redox
Redox (/ˈrɛdɒks/ RED-oks, /ˈriːdɒks/ REE-doks, reduction–oxidation[2] or oxidation–reduction[3]: 150 ) is a type of chemical reaction in which the oxidation states of the reactants change.[5] In electrochemical reactions the oxidation and reduction processes do occur simultaneously but are separated in space.Ultimately, the meaning was generalized to include all processes involving the loss of electrons or the increase in the oxidation state of a chemical species.Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents.[3]: 288 The electrochemist John Bockris proposed the words electronation and de-electronation to describe reduction and oxidation processes, respectively, when they occur at electrodes.[17] They have not been widely adopted by chemists worldwide,[citation needed] although IUPAC has recognized the terms electronation[18] and de-electronation.[19] Redox reactions can occur slowly, as in the formation of rust, or rapidly, as in the case of burning fuel.[citation needed] Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis.The process of electroplating uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated automotive parts, silver plating cutlery, galvanization and gold-plated jewelry.Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen.The redox state is reflected in the balance of several sets of metabolites (e.g., lactate and pyruvate, beta-hydroxybutyrate and acetoacetate), whose interconversion is dependent on these ratios.Once formed, these anion free radicals reduce molecular oxygen to superoxide and regenerate the unchanged parent compound.The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide.The main chemical reaction producing the molten iron is:[26] Electron transfer reactions are central to myriad processes and properties in soils, and redox potential, quantified as Eh (platinum electrode potential (voltage) relative to the standard hydrogen electrode) or pe (analogous to pH as -log electron activity), is a master variable, along with pH, that controls and is governed by chemical reactions and biological processes.[27] Later work built on this foundation, and expanded it for understanding redox reactions related to heavy metal oxidation state changes, pedogenesis and morphology, organic compound degradation and formation, free radical chemistry, wetland delineation, soil remediation, and various methodological approaches for characterizing the redox status of soils.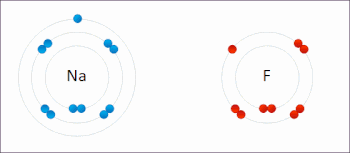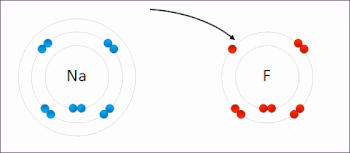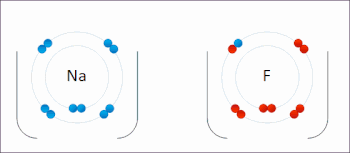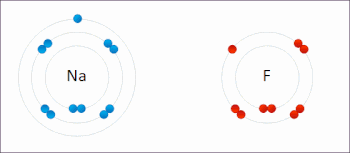
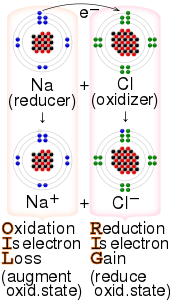







Redox (disambiguation)Sodiumfluorinesodium fluorideglycerolpotassium permanganatechemical reactionoxidation statesreactantselectronsElectron-transfersubstraterustinghydrogenationtransfer of hydrogen atomsportmanteaureducing agentoxidizing agenthalf-reactionelectrochemical reactionssubstancesoxidizing agentselectron acceptorMnO−4Cr2O2−7electronegativeNitric acidinternationalpictogramreducing agentselectron donorcharge transfer complexesmetal oxideAntoine Lavoisierchemical specieselectronbiochemistryhydride ionElectropositivemetalslithiummagnesiumaluminiumLiAlH4carbonylalcoholselectrochemistJohn Bockriselectrodesprotonationdeprotonationinner sphere electron transferouter sphere electron transferionization energieselectrode potentialvoltagestandard conditionselectrochemical cellcathodestandard hydrogen electrodehydrogenhalf-reactionsprotonshydrogen fluorideGalvanic cellcoppercopper(II) sulfatenitratenitrogendenitrificationcombustionhydrocarbonsinternal combustion enginecarbon dioxidecarbon monoxideenergycarbonorganic chemistryalcoholaldehydeketonecarboxylic acidperoxideiron(III) oxideiron(III) oxidesiron(III) oxide-hydroxidepyritecorrosioniron oxideshydrogen peroxidedisproportionationthiosulfatesulfur dioxideCathodic protectionsacrificial anodegalvanizedammoniaelectrosynthesissmeltingelectroplatingchrome-platedautomotivecutlerygalvanizationgold-platedjewelryascorbic acidreduced formVitamin Cdehydroascorbic acidoxidized formbiologicalassimilatedCellular respirationglucoseoxygenPhotosynthesislight energyBiological energysugarsnicotinamide adenine dinucleotideproton gradientadenosine triphosphatemitochondriaMembrane potentialFree radical