Collision theory
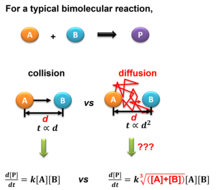
Collision theory is a principle of chemistry used to predict the rates of chemical reactions.Collision theory was proposed independently by Max Trautz in 1916[1] and William Lewis in 1918.Collision theory was initially developed for the gas reaction system with no dilution.[4] In this model, Fick's flux at the infinite time limit is used to mimic the particle speed of the collision theory.[5] The rate for a bimolecular gas-phase reaction, A + B → product, predicted by collision theory is[6] where: The unit of r(T) can be converted to mol⋅L−1⋅s−1, after divided by (1000×NA), where NA is the Avogadro constant.As molecules are quantum-mechanical many-particle systems of electrons and nuclei based upon the Coulomb and exchange interactions, generally they neither obey rotational symmetry nor do they have a box potential.Therefore, more generally the cross section is defined as the reaction probability of a ray of A particles per areal density of B targets, which makes the definition independent from the nature of the interaction between A and B. Consequently, the radiusTherefore, the rate of a bimolecular reaction for ideal gases will be where: The product zρ is equivalent to the preexponential factor of the Arrhenius equation.The reason for this is that particles have been supposed to be spherical and able to react in all directions, which is not true, as the orientation of the collisions is not always proper for the reaction.For example, in the hydrogenation reaction of ethylene the H2 molecule must approach the bonding zone between the atoms, and only a few of all the possible collisions fulfill this requirement.The deviation from unity can have different causes: the molecules are not spherical, so different geometries are possible; not all the kinetic energy is delivered into the right spot; the presence of a solvent (when applied to solutions), etc.Collision theory can be applied to reactions in solution; in that case, the solvent cage has an effect on the reactant molecules, and several collisions can take place in a single encounter, which leads to predicted preexponential factors being too large.Theoretical models to calculate the collision frequency in solutions have been proposed by Marian Smoluchowski in a seminal 1916 publication at the infinite time limit,[4] and Jixin Chen in 2022 at a finite-time approximation.[5] A scheme of comparing the rate equations in pure gas and solution is shown in the right figure.For the diffusive collision, at the infinite time limit when the molecular flux can be calculated from the Fick's laws of diffusion, in 1916 Smoluchowski derived a collision frequency between molecule A and B in a diluted solution:[4] where: or where: There have been a lot of extensions and modifications to the Smoluchowski model since it was proposed in 1916.In 2022, Chen rationales that because the diffusive flux is evolving over time and the distance between the molecules has a finite value at a given concentration, there should be a critical time to cut off the evolution of the flux that will give a value much larger than the infinite solution Smoluchowski has proposed.This hypothesis yields a fractal reaction kinetic rate equation of diffusive collision in a diluted solution:[5] where:
Reaction rateconcentrationchemistrychemical reactionsreactantactivation energytransition state theoryMax TrautzWilliam Lewischemical kineticsdiffusionBrownian motionFick's laws of diffusionSmoluchowski coagulation equationMarian Smoluchowskinumber densitycollision frequencysteric factortemperaturegas constantAvogadro constantcross sectionBoltzmann constantreduced masskinetic theoryaverage velocityroot mean squaretwo-body problemcenter of masspreexponential factorArrhenius equationrate equationhydrogenationethylenefrequency factorharpoon reactionselectronsrate constantsentropicC2H5BrethanolC2H5O−methanol4-CH3C6H4O−acetoneC2H2Cl4mean free pathfractalTwo-dimensional gasBibcodereaction mechanismsNucleophilic substitutionsUnimolecular nucleophilic substitutionBimolecular nucleophilic substitutionNucleophilic aromatic substitutionNucleophilic internal substitutionNucleophilic acyl substitutionElectrophilic substitutionsElectrophilic aromatic substitutionElimination reactionsUnimolecular eliminationE1cB-eliminationBimolecular eliminationEi eliminationAddition reactionsElectrophilic additionNucleophilic additionFree-radical additionCycloadditionOxidative additionIntramolecular reactionIsomerizationPhotodissociationLindemann–Hinshelwood mechanismRRKM theoryElectron/Proton transferHarpoon reactionGrotthuss mechanismMarcus theoryInner sphere electron transferOuter sphere electron transferSolvent effectsCage effectMatrix isolationElementary reactionReaction dynamicsReactive intermediateRadical (chemistry)MolecularityStereochemistryCatalysisArrow pushingPotential energy surfaceMore O'Ferrall–Jencks plotEquilibrium constantRate-determining stepReaction coordinateEnergy profile (chemistry)Activated complexEyring equationMichaelis–Menten kineticsDiffusion-controlled reaction