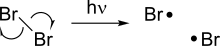
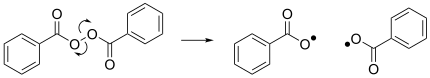
Radical (chemistry)
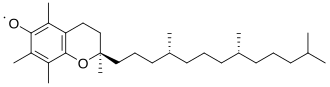
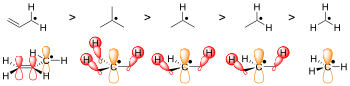
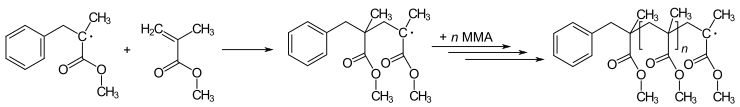
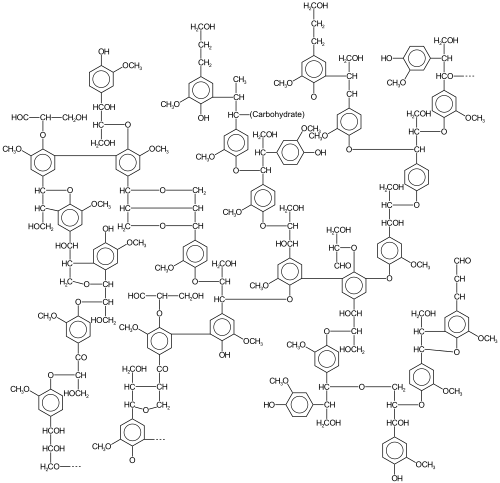
In living organisms, the radicals superoxide and nitric oxide and their reaction products regulate many processes, such as control of vascular tone and thus blood pressure.Radicals are formed from spin-paired molecules through homolysis of weak bonds or electron transfer, also known as reduction.[3] Because breaking a chemical bond requires energy, homolysis occurs under the addition of heat or light.To achieve this reaction, the C-H bond of the H-atom donor must be weak, which is rarely the case in organic compounds.However, these radicals are kinetically transient because they can undergo rapid, diffusion-limited dimerization, resulting in a lifetime that is less than a few nanoseconds.Most simply, the greater the steric hindrance the more difficult it is for reactions to take place, and the radical form is favored by default.[3] TEMPO, or (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl, is too sterically hindered by the additional methyl groups to react making it stable enough to be sold commercially in its radical form.The tocopherol radical itself is insufficiently stable for isolation, but the parent molecule is a highly effective hydrogen-atom donor.Singlet oxygen, the lowest-energy non-radical state of dioxygen, is less stable than the diradical due to Hund's rule of maximum multiplicity.The relative stability of the oxygen diradical is primarily due to the spin-forbidden nature of the triplet-singlet transition required for it to grab electrons, i.e., "oxidize".The diradical state of oxygen also results in its paramagnetic character, which is demonstrated by its attraction to an external magnet.[12] Diradicals can also occur in metal-oxo complexes, lending themselves for studies of spin forbidden reactions in transition metal chemistry.It also means molecular oxygen is relatively unreactive at room temperature except in the presence of a catalytic heavy atom such as iron or copper.This prevents the combustion from initiating in an uncontrolled manner or in unburnt residues (engine knocking) or premature ignition (preignition).Drying oils and alkyd paints harden due to radical crosslinking initiated by oxygen from the atmosphere.In the lower atmosphere, important radical are produced by the photodissociation of nitrogen dioxide to an oxygen atom and nitric oxide (see eq.1.1 below), which plays a key role in smog formation—and the photodissociation of ozone to give the excited oxygen atom O(1D) (see eq.In the upper atmosphere, the photodissociation of normally unreactive chlorofluorocarbons (CFCs) by solar ultraviolet radiation is an important source of radicals (see eq.[17] The classic free-radical syndrome, the iron-storage disease hemochromatosis, is typically associated with a constellation of free-radical-related symptoms including movement disorder, psychosis, skin pigmentary melanin abnormalities, deafness, arthritis, and diabetes mellitus.Similarly, the process of mitohormesis suggests that repeated exposure to radicals may extend life span.Furthermore, there is good evidence indicating that bilirubin and uric acid can act as antioxidants to help neutralize certain radicals.Too much bilirubin, though, can lead to jaundice, which could eventually damage the central nervous system, while too much uric acid causes gout.ROS form as a natural by-product of the normal metabolism of oxygen and have important roles in cell signaling.Excessive amounts of these radicals can lead to cell injury and death, which may contribute to many diseases such as cancer, stroke, myocardial infarction, diabetes and major disorders.This only occurred when it was combined with other ingredients commonly found in sunscreens, like titanium oxide and octyl methoxycinnamate.known as type II photooxygenation reactions after Dexter energy transfer (triplet-triplet annihilation) from natural triplet dioxygenTypical chemical transformations with this singlet dioxygen species involve, among others, conversion of cellulosic biowaste into new poylmethine dyes.[24] In chemical equations, radicals are frequently denoted by a dot placed immediately to the right of the atomic symbol or molecular formula as follows: Radical reaction mechanisms use single-headed arrows to depict the movement of single electrons: The homolytic cleavage of the breaking bond is drawn with a "fish-hook" arrow to distinguish from the usual movement of two electrons depicted by a standard curly arrow.Following recent nomenclature revisions, a part of a larger molecule is now called a functional group or substituent, and "radical" now implies "free".[25][26] In most fields of chemistry, the historical definition of radicals contends that the molecules have nonzero electron spin.











Free-radical theory of agingFree radical (disambiguation)Moiety (chemistry)hydroxyl radicalLewis structureLewis dot structureHydroxidechemistrymoleculeunpaired valence electronchemically reactivedimerizetriplet oxygentriplet carbeneredox reactionsionizing radiationelectrolysiscombustionatmospheric chemistrypolymerizationplasmabiochemistrysuperoxidenitric oxideredox signalingsolvent cagehomolysisadditionbond dissociation energylithium naphthalenereductionsdelocalizednaphthenidesanthracenidesketylsHydrogen abstractionAllylicdrying oilslinoleic acidfree-radical additionsradical polymerizationplasticsα-tocopherol2,2,6,6-tetramethylpiperidinyloxylmolecular orbital theoryhyperconjugationN-hydroxypiperidinevitamin EtriphenylmethylFremy's saltthiazylresonance stabilizationbreaking of covalent bondsbond dissociation energieskJ/molDiradicalsDioxygenSinglet oxygenHund's rule of maximum multiplicityspin-forbiddenmetal-oxo complexesspin forbidden reactionstransition metalCarbenespersistent carbenesnitrenesbutaneSwan bandsoxygendiradicalground statetripletsingletenergy barrierforbiddenflammabilitytetraethyl leadengine knockingpreignitionhydroperoxyl radicalorganic hydroperoxideshydroxyl radicalsmethyl methacrylatePoly(methyl methacrylate)radical additionliving radical polymerizationhydroperoxideOxygen cycleOzone-oxygen cyclePhotodissociationnitrogen dioxidechlorofluorocarbonsultraviolet radiationchlorineozone depletionozone layerrefrigerantsDioxygen in biological reactionsdeoxyadenosyl radicalligningranulocytesmacrophagescell signalling13-hydroxyoctadecadienoic acids9-hydroxyoctadecadienoic acidsParkinson's diseasedeafnessschizophreniaAlzheimer'shemochromatosismelaninfree-radical theoryaging processhormesisenzymessuperoxide dismutasecatalaseglutathione peroxidaseglutathione reductaseantioxidantsvitamin Avitamin Cpolyphenol antioxidantsbilirubinuric acidred blood cells