Hydroxylamine
[5] Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate.[citation needed] A direct lab synthesis of hydroxylamine from molecular nitrogen in water plasma was demonstrated in 2024.[13] Hydroxylamine reacts with electrophiles, such as alkylating agents, which can attach to either the oxygen or the nitrogen atoms: The reaction of NH2OH with an aldehyde or ketone produces an oxime.High concentrations of hydroxylamine are used by biologists to introduce mutations by acting as a DNA nucleobase amine-hydroxylating agent.[23] In is thought to mainly act via hydroxylation of cytidine to hydroxyaminocytidine, which is misread as thymidine, thereby inducing C:G to T:A transition mutations.[24] But high concentrations or over-reaction of hydroxylamine in vitro are seemingly able to modify other regions of the DNA & lead to other types of mutations.[25] Practically, it has been largely surpassed by more potent mutagens such as EMS, ENU, or nitrosoguanidine, but being a very small mutagenic compound with high specificity, it found some specialized uses such as mutation of DNA packed within bacteriophage capsids,[26] and mutation of purified DNA in vitro.[30] Cytochrome P460, an enzyme found in the ammonia-oxidizing bacteria Nitrosomonas europea, can convert hydroxylamine to nitrous oxide, a potent greenhouse gas.




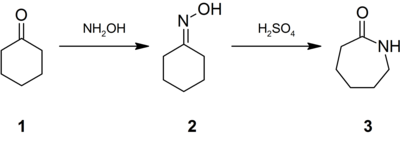

hemiaminalIUPAC namePreferred IUPAC nameCAS NumberChEMBLChemSpiderECHA InfoCardEC NumberGmelin ReferencePubChemRTECS numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterAcidityBasicityCoordination geometryMolecular shapeTrigonal pyramidalHeat capacityStd molarentropyStd enthalpy offormationGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointAutoignitiontemperatureSafety data sheetHydroxylammonium chlorideHydroxylammonium nitrateHydroxylammonium sulfateAmmoniaHydrazineHydrogen peroxideN,O-DimethylhydroxylamineN,N-DiethylhydroxylamineHydroxylamine-O-sulfonic acidstandard stateinorganic compoundhygroscopiccrystalsaqueous solutionNylon-6oxidationnitrificationWilhelm Clemens Lossenhydrochloric acidethyl nitrateLobry de Bruyncoordination complexcationsbiochemistrysulfuric acidhydrogenationnitric oxideplatinumcatalystsRaschig processaqueousammonium nitritereducedHSO−3[NH4]+hydrolyzedliquid ammoniaAmmonium sulfateNO−2SO2−4[NH3OH]ClCH3(CH2)3OHJulius Tafelhydrochloridesulfateelectrolytic reductionnitric acidnitrous acidpotassium nitritebisulfitenitromethanehydroxylamine hydrochloridecarbon monoxidemolecular nitrogenwater plasmaelectrophilesalkylating agentsoxygennitrogenaldehydeketonedimethylglyoximeligandschlorosulfonic aciddetonatorisomerisationamine oxideHydroxamic acidsubstitutedglycosidic bondcalicheamicinN,O‑DimethylhydroxylamineWeinreb amidesnitronesorganylaminesbenzoyl peroxidenitroneAlkylatingCope reactionBeckmann rearrangementcyclohexanone oximeNylon 6caprolactammutationsnucleobasecytidineErnst FreesenitrosoguanidinebacteriophageHoechst