Alkylation
Examples include the use of organometallic compounds such as Grignard (organomagnesium), organolithium, organocopper, and organosodium reagents.The largest example of this takes place in the alkylation units of petrochemical plants, which convert low-molecular-weight alkenes into high octane gasoline components.[3] On a laboratory scale the Friedel–Crafts reaction uses alkyl halides, as these are often easier to handle than their corresponding alkenes, which tend to be gasses.Industry often relies on green chemistry methods involving alkylation of amines with alcohols, the byproduct being water.Similar reactions occur when tertiary phosphines are treated with alkyl halides, the products being phosphonium salts.[citation needed] Diazomethane is a popular methylating agent in the laboratory, but it is too hazardous (explosive gas with a high acute toxicity) to be employed on an industrial scale without special precautions.This mechanism of toxicity is relevant to the function of anti-cancer drugs in the form of alkylating antineoplastic agents.Some chemical weapons such as mustard gas (sulfide of dichloroethyl) function as alkylating agents.The product, called "alkylate", is composed of a mixture of high-octane, branched-chain paraffinic hydrocarbons (mostly isoheptane and isooctane).Alkylate is a premium gasoline blending stock because it has exceptional antiknock properties and is clean burning.By combining fluid catalytic cracking, polymerization, and alkylation, refineries can obtain a gasoline yield of 70 percent.

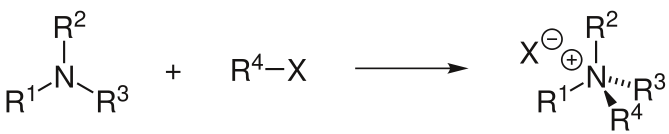


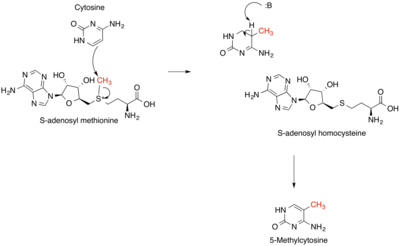
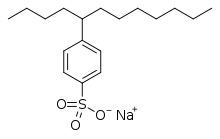

chemical reactioncarbocationfree radicalcarbanioncarbenereagentsnucleophilicelectrophilicisobutaneolefinspetroleumchemotherapyalkylating antineoplastic agentselectrophilecovalent bondwork-uporganometallic compoundsGrignard (organomagnesium)organolithiumorganocopperorganosodiumcarbonyl grouphalidecatalystSuzuki couplingsKumada couplingLigandalkylation unitsalkenesgasolinephenolslinear alkylbenzenessurfactantsantioxidantsAmberlystLewis acidsFriedel–Crafts reactionalkyl halidesaluminium trichloridegreen chemistryHydroaminationMenshutkin reactiontertiary aminequaternary ammonium saltalkyl halideThiolsthioethersthiol-ene reactionsulfonium ionsAlcoholsethersWilliamson ether synthesisspectator ionethoxylationEthylene oxideoxidative additionCativa processacetic acidmethyl iodidecross coupling reactionsTriethyloxonium tetrafluoroboratecationTrimethyloxonium tetrafluoroborateDimethyl sulfateDiazomethanemethylating agenttrimethylsilyldiazomethanechemical weaponsmustard gasFriedel-Crafts alkylationBrønsted acidsSilicotungstic acidethyl acetateethylenemethylationDNA damagenitrogenous basesBase Excision RepairDNA methylationSAM cofactorethylbenzenestyrenecumenephenolacetonelinear alkylbenzene sulfonateslinear alkylbenzene sulfonatealkylation unit2,4-dimethylpentaneoil refinerypropenebutenesolid acidscarbocationsoctaneparaffinichydrocarbonsisoheptaneisooctanefluid catalytic crackingsulfuric acidhydrofluoric acidIonic liquidsdemethylationsmethyl ethersmethyl aminesHydrodealkylationTransalkylationAlkynylationBibcodeOrganic SynthesesMedical Subject Headings