Cholangiocarcinoma
[6] The most common physical indications of cholangiocarcinoma are abnormal liver function tests, jaundice (yellowing of the eyes and skin occurring when bile ducts are blocked by tumor), abdominal pain (30–50%), generalized itching (66%), weight loss (30–50%), fever (up to 20%), and changes in the color of stool or urine.In the Western world, the most common of these is primary sclerosing cholangitis (PSC), an inflammatory disease of the bile ducts which is closely associated with ulcerative colitis (UC).[13] Epidemiologic studies have suggested that the lifetime risk of developing cholangiocarcinoma for a person with PSC is on the order of 10–15%,[14] although autopsy series have found rates as high as 30% in this population.[28] Congenital liver abnormalities, such as Caroli disease (a specific type of five recognized choledochal cysts), have been associated with an approximately 15% lifetime risk of developing cholangiocarcinoma.They are often surrounded by a brisk fibrotic or desmoplastic tissue response; in the presence of extensive fibrosis, it can be difficult to distinguish well-differentiated cholangiocarcinoma from normal reactive epithelium.Although ERCP is an invasive procedure with attendant risks, its advantages include the ability to obtain biopsies and to place stents or perform other interventions to relieve biliary obstruction.[12] Endoscopic ultrasound can also be performed at the time of ERCP and may increase the accuracy of the biopsy and yield information on lymph node invasion and operability.[12] General guidelines for operability include:[68][69] Cholangiocarcinoma is considered to be an incurable and rapidly lethal disease unless all the tumors can be fully resected (cut out surgically).[12] In 2008, the Mayo Clinic reported significant success treating early bile duct cancer with liver transplantation using a protocolized approach and strict selection criteria.Both positive[74][75] and negative[11][76][77] results have been reported with adjuvant radiation therapy in this setting, and no prospective randomized controlled trials have been conducted as of March 2007.Chemotherapy has been shown in a randomized controlled trial to improve quality of life and extend survival in people with inoperable cholangiocarcinoma.[88] A small pilot study suggested possible benefit from the tyrosine kinase inhibitor erlotinib in people with advanced cholangiocarcinoma.[6] Infigratinib (Truseltiq) is a tyrosine kinase inhibitor of fibroblast growth factor receptor (FGFR) that was approved for medical use in the United States in May 2021.[91] Pemigatinib (Pemazyre) is a kinase inhibitor of fibroblast growth factor receptor 2 (FGFR2) that was approved for medical use in the United States in April 2020.[92] It is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement as detected by an FDA-approved test.The FDA approved ivosidenib in August 2021 for adults with previously treated, locally advanced or metastatic cholangiocarcinoma with an isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test.For non-resectable cases, the five-year survival rate is 0% where the disease is inoperable because distal lymph nodes show metastases,[96] and less than 5% in general.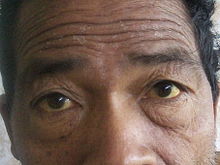





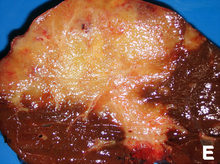


Micrographliver cellsH&E stainSpecialtyOncologySymptomsAbdominal painyellowish skinweight lossgeneralized itchingRisk factorsPrimary sclerosing cholangitisulcerative colitisliver flukesDiagnostic methodmicroscopeSurgical resectionchemotherapyradiation therapystentingliver transplantationPrognosiscancerbile ductsbiliary tractgallbladder cancerampulla of Vatercirrhosishepatitis Chepatitis Bblood testsmedical imagingendoscopyadenocarcinomaglandspalliative treatmentscommon bile duct5-year survivalWestern worldSoutheast AsiaThailandjaundicescleral icterusliver function testsBlood tests of liver functionbilirubinalkaline phosphatasegamma glutamyl transferasetransaminaseinflammationparenchymaClonorchis sinensisliver flukeinflammatory diseaseDNA repairDNA damagebile acidsparasitic liver diseasesOpisthorchis viverriniIntegrated Opisthorchiasis Control Programalcoholic liver diseaseHIV infectionHelicobacter bilisHelicobacter hepaticusCongenitalCaroli diseasecholedochal cystsLynch syndromecholelithiasishepatolithiasisThorotrastthorium dioxideradiologic contrast mediumcarcinogenicitycommon hepatic ductKlatskin tumorpluripotentstem cellhyperplasiametaplasiadysplasiacarcinomacolon cancerChronic inflammationHistologicallyundifferentiated to well-differentiatedfibroticdesmoplasticepitheliumimmunohistochemicalmalignantbenigncytokeratinscarcinoembryonic antigenmucinsadenocarcinomashepatocytesportal triadbile ductCA19-9sensitivespecificscreeningimaging methodsCT scanUltrasoundbiliary treeComputed tomographyEndoscopic retrograde cholangiopancreatographyendoscopicgastroenterologistbiopsiesstentsEndoscopic ultrasoundlymph nodepercutaneous transhepatic cholangiographyMagnetic resonance cholangiopancreatographynon-invasiveSurgical explorationbiopsyLaparoscopylaparotomyImmunohistochemistryhepatocellular carcinomaCytological scrapingsstaging systems