Fertilizer
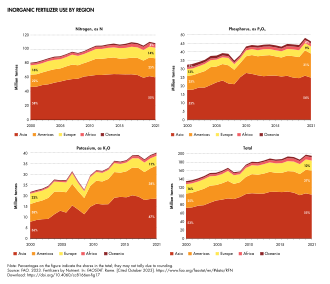
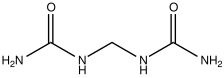
[1] For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients.Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods.However, starting in the 19th century, after innovations in plant nutrition, an agricultural industry developed around synthetically created agrochemical fertilizers.This transition was important in transforming the global food system, allowing for larger-scale industrial agriculture with large crop yields.Fertilizer use along with agriculture allowed some of these early societies a critical advantage over their neighbors, leading them to become dominant cultures in their respective regions (P Bellwood - 2023[6])[7].[1] The scientific research of plant nutrition started well before the work of German chemist Justus von Liebig although his name is most mentioned as the "father of the fertilizer industry".[8] Nicolas Théodore de Saussure and scientific colleagues at the time were quick to disprove the simplifications of von Liebig.[14] It is estimated that a third of annual global food production uses ammonia from the Haber–Bosch process and that this supports nearly half the world's population.[15][16] After World War II, nitrogen production plants that had ramped up for wartime bomb manufacturing were pivoted towards agricultural uses.[26] Nitrite-oxidizing bacteria, especially Nitrobacter, oxidize nitrite (NO−2) to nitrate (NO−3), which is extremely soluble and mobile and is a major cause of eutrophication and algal bloom.Most fertilizers are labeled according to this N-P-K convention, although Australian convention, following an N-P-K-S system, adds a fourth number for sulfur, and uses elemental values for all values including P and K.[31] Micronutrients are consumed in smaller quantities and are present in plant tissue on the order of parts-per-million (ppm), ranging from 0.15 to 400 ppm or less than 0.04% dry matter.Iron presents special problems because it converts to insoluble (bio-unavailable) compounds at moderate soil pH and phosphate concentrations.Deposits of sodium nitrate (NaNO3) (Chilean saltpeter) are also found in the Atacama Desert in Chile and was one of the original (1830) nitrogen-rich fertilizers used.Potash is soluble in water, so the main effort in producing this nutrient from the ore involves some purification steps, e.g., to remove sodium chloride (NaCl) (common salt).[40] There are three major routes for manufacturing NPK fertilizers (named for their main ingredients: nitrogen (N), phosphorus (P), and potassium (K)): Step 2.Organic fertilizers can also describe commercially available and frequently packaged products that strive to follow the expectations and restrictions adopted by "organic agriculture" and "environmentally friendly" gardening – related systems of food and plant production that significantly limit or strictly avoid the use of synthetic fertilizers and pesticides.It is an immature form of coal and improves the soil by aeration and absorbing water but confers no nutritional value to the plants.Organic fertilizers such as composts and manures may be distributed locally without going into industry production, making actual consumption more difficult to quantify.[50] The diagram below shows fertilizer consumption by the European Union (EU) countries as kilograms per hectare (pounds per acre).During summer, urea is often spread just before or during rain to minimize losses from volatilization (a process wherein nitrogen is lost to the atmosphere as ammonia gas).[3] The use of fertilizer has also led to a number of direct environmental consequences: agricultural runoff which leads to downstream effects like ocean dead zones and waterway contamination, soil microbiome degradation,[62] and accumulation of toxins in ecosystems.The main contributor to eutrophication is phosphate, which is normally a limiting nutrient; high concentrations promote the growth of cyanobacteria and algae, the demise of which consumes oxygen.[65] The nitrogen-rich compounds found in fertilizer runoff are the primary cause of serious oxygen depletion in many parts of oceans, especially in coastal zones, lakes and rivers.[74][75] In cases where eutrophication can be reversed, it may nevertheless take decades[76] and significant soil management[77] before the accumulated nitrates in groundwater can be broken down by natural processes.[79][80][81] The excessive use of nitrogen-containing fertilizers (be they synthetic or natural) is particularly damaging, as much of the nitrogen that is not taken up by plants is transformed into nitrate which is easily leached.[84] Run-off can lead to fertilizing blooms of algae that use up all the oxygen and leave huge "dead zones" behind where other fish and aquatic life can not live.[103][104][105] Where high annual rates of phosphorus fertilizer are used, this can result in uranium-238 concentrations in soils and drainage waters that are several times greater than are normally present.These highly water-soluble fertilizers are used in the plant nursery business and are available in larger packages at significantly less cost than retail quantities.[128] Nitrous oxide emissions by humans, most of which are from fertilizer, between 2007 and 2016 have been estimated at 7 million tonnes per year,[129] which is incompatible with limiting global warming to below 2 °C.It has a global warming potential 296 times larger than an equal mass of carbon dioxide and it also contributes to stratospheric ozone depletion.


















Bath salts (drug)Plant food (disambiguation)farmermanuresoil fertilityAmerican EnglishBritish Englishplant nutrientsliming materialssoil amendmentsindustriallynitrogenphosphoruspotassiumrock flourcompostanimal manurehuman manurecrop rotationsfish processing wastebloodmealanimal slaughterplant nutritionagricultural industryagrochemical fertilizersglobal food systemindustrial agricultureNitrogen-fixingHaber processconventional food systemsGreen Revolutionwater pollutioneutrophicationcarboncontamination and pollution of soilsustainable agriculturepesticideenvironmental damageHistory of fertilizermanuresSalamancaJustus von LiebigNicolas Théodore de SaussureCarl Ludwig SprengerHermann HellriegelJohn Bennet LawesentrepreneurJoseph Henry GilbertInstitute of Arable Crops ResearchBirkeland–Eyde processnitric acidnitrogen fixationnitrateRjukanNotoddenhydroelectric powerOstwald processmethanenatural gastonneshectarephosphateYara Internationalproportionscalciummagnesiumsulfurcoppermanganesemolybdenumsiliconcobaltvanadiumcompoundsdry mattercarbon dioxideatmosphereproteinsamide bondsamino acidspyrimidictetrapyrrolicchlorophylllegumesammoniagenetic codelipidsphospholipidslipidic double layercell membranesenzymatic reactionshydrolysisbacteriaureasecatalyzesammoniumbicarbonateAmmonia-oxidizing bacteriaNitrosomonasoxidizenitritenitrificationNitrite-oxidizing bacteriaNitrobactersolublealgal bloomAmmonium nitrateCalcium ammonium nitratelimestonedolomiteCalcium nitratesuperphosphatesphosphogypsumTriple superphosphatemuriate of potashmonoammonium phosphatediammonium phosphateLabeling of fertilizerNPK ratingMicronutrientsparts-per-millionchelate complexsugar beetsbiological processesproducedHaber–Bosch process

