Basketane
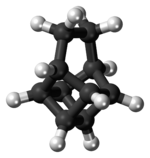
[3] A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.[4] In the year 1989 and before the synthesis of basketane, historic chemists were intrigued by the structural make-up of molecules, specifically those in objects seen in everyday life.[5] Using supramolecular chemistry, molecules such as cubane and basketane were named according to their corresponding shape and historically revealed certain characteristics and personal motives of chemists at that time.The acid is decarboxylated with a modified Hunsdiecker reaction to a dibromide 7, which is reductively debrominated with tributyltin hydride to basketane (8) at a 11% yield relative to the starting material cyclohexa-1,3-diene.[12] Basketane absorbs an equivalent amount of hydrogen gas in the presence of palladium on carbon, After some initial confusion, it was shown that the C3-C4 bond is hydrogenolyzed to give the dihydrobasketane tetracyclo[4.4.0.02,5.03,8]decane.
Preferred IUPAC nameCAS NumberChemSpiderPubChemCompTox DashboardSMILESChemical formulaMolar massstandard statepolycyclicalkanebaskethydrocarbondopingdiamondchemistsmoleculessupramolecular chemistrychemical nomenclaturecarbon-carbon bondsDiels–Alder reactioncyclooctatetraenemaleic anhydridephotoisomerizeslead tetraacetate1,4-benzoquinonecyclohexa-1,3-dieneFavorskii rearrangementsodium hydroxideHunsdiecker reactiontributyltin hydrideradicalstrainring expansionring systemsCyclobutylmethylfree energy barrierskcal/molstrained ringsheat of combustionvalence isomerizationSilver perchloratepalladium on carbontwistaneCubaneList of chemical compounds with unusual namesChem. Rev.Tetrahedron Lett.Hughes Aircraft Co.J. Am. Chem. Soc.HydrocarbonsAlkanesMethaneEthanePropaneButanePentaneHexaneHeptaneOctaneNonaneDecaneIsobutaneIsopentane3-MethylpentaneNeopentaneIsohexaneIsoheptaneIsooctaneIsononaneCycloalkanesCyclopropaneCyclobutaneCyclopentaneCyclohexaneCycloheptaneCyclooctaneCyclononaneCyclodecaneMethylcyclopropaneMethylcyclopentaneMethylcyclohexaneHousaneNorbornaneDecalinAdamantaneDiamondoidSteranePrismaneDodecahedraneChurchanePagodaneUnsaturatedAlkenesEthenePropeneButenePenteneHexeneHepteneOcteneNoneneDeceneIsobuteneIsopenteneAlkynesEthynePropyneButynePentyneHexyneHeptyneOctyneNonyneDecyneCycloalkenesCyclopropeneCyclobuteneCyclopenteneCyclohexeneCyclohepteneCycloocteneCyclononeneCyclodeceneMethylcyclopropene