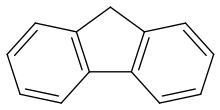
Fluorene
It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene.For commercial purposes it is obtained from coal tar,[3] where it was discovered and named by Marcellin Berthelot in 1867.[8] Fluorene can be found after the incomplete combustion of plastics such as PS, PE and PVC.[10]) Deprotonation gives the stable fluorenyl anion, nominally C13H9−, which is aromatic and has an intense orange colour.The purification of fluorene exploits its acidity and the low solubility of its sodium derivative in hydrocarbon solvents.
FluoraneFluorineFluoroneFluorenonePreferred IUPAC nameCAS NumberChEMBLChemSpiderECHA InfoCardEC NumberPubChemRTECS numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilityAcidityMagnetic susceptibilityNFPA 704Flash pointSafety data sheetstandard stateorganic compoundnaphthalenefluorescencecoal tarMarcellin Berthelotpolycyclic aromatic hydrocarbondiphenylmethanehypophosphorous acidiodineincomplete combustionplasticsaromaticnucleophileElectrophilespotassiumdioxaneligandscyclopentadienideKaminsky precatalystsyndiotacticpolypropylene9-Fluorenylmethyl chloroformateprotecting grouppeptide synthesisPolyfluoreneelectrically conductiveelectroluminescentluminophoreorganic light-emitting diodesCataCXium F sulfFluorenolIndecainidePD-137889CarbazoleDibenzothiopheneMerck IndexInternational Union of Pure and Applied ChemistryThe Royal Society of ChemistryUllmann's Encyclopedia of Industrial ChemistryJohn IballBibcodePolycyclic aromatic hydrocarbonsButaleneAzuleneAcenaphtheneAcenaphthyleneAnthracenePhenalenePhenanthreneBenz[a]anthraceneBenzo[a]fluoreneBenzo[c]fluoreneBenzo[c]phenanthreneChryseneFluoranthenePyreneTetraceneTriphenyleneTricyclobutabenzeneBenz[e]acephenanthryleneBenzopyreneBenzo[a]pyreneBenzo[e]pyrene6H-Benzo[cd]pyrene(Olympicene)Benzo[a]fluorantheneBenzo[b]fluorantheneBenzo[j]fluorantheneBenzo[k]fluoranthenetrans-BicaliceneDibenz[a,h]anthraceneDibenz[a,j]anthracenePentacenePerylenePiceneTetraphenyleneAnthanthreneBenzo[ghi]peryleneCorannuleneDibenzopyrenesHexaceneTrianguleneZethreneCoroneneDicoronyleneDiindenoperyleneHeptaceneHexabenzocoroneneHexa-cata-KekuleneOvaleneRubiceneRubreneSumaneneSuperphenaleneTrinaphthyleneTruxeneCirculeneCyclaceneHelicene