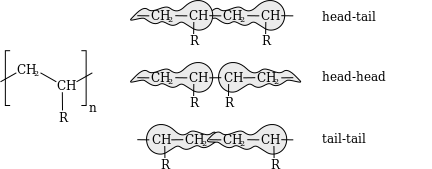
Tacticity
When the stereochemistry of a macromolecule is considered to be a Bernoulli process, the triad composition can be calculated from the probability Pm of a diad being m type.[5]: 357 The definition of tetrads and pentads introduce further sophistication and precision to defining tacticity, especially when information on long-range ordering is desirable.[citation needed] Tacticity measurements obtained by carbon-13 NMR are typically expressed in terms of the relative abundance of various pentads within the polymer molecule, e.g. mmmm, mrrm.The primary convention for expressing tacticity is in terms of the relative weight fraction of triad or higher-order components, as described above.[3] With the aid of spectroscopic techniques such as NMR, it is possible to pinpoint the composition of a polymer in terms of the percentages for each triad.[citation needed] The two materials have very different properties because the irregular structure of the atactic version makes it impossible for the polymer chains to stack in a regular fashion: whereas syndiotactic PS is a semicrystalline material, the more common atactic version cannot crystallize and forms a glass instead.[citation needed] In eutactic macromolecules, substituents may occupy any specific (but potentially complex) sequence of positions along the chain.In a regular macromolecule all monomer units are normally linked in a head to tail configuration so that all β-substituents are separated by three carbon atoms.This technique enables quantification of the tacticity distribution by comparison of peak areas or integral ranges corresponding to known diads (r, m), triads (mm, rm+mr, rr) and/or higher order n-ads, depending on spectral resolution.In cases of limited resolution, stochastic methods such as Bernoullian or Markovian analysis may also be used to fit the distribution and predict higher n-ads and calculate the isotacticity of the polymer to the desired level.







ArchitectureDegradationMark–Houwink theoryFlory–Huggins solution theoryCoil–globule transitionSynthesisRadical polymerizationNitroxide-mediated radical polymerizationStep polymerizationCondensation polymerizationAddition polymerizationClassificationPolyolefinPolyethylenePolypropylenePolyisobutylenePolyurethanePolyesterPolycarbonateVinyl polymersPolystyreneHomopolymerCopolymerHydrogelsSelf-healing hydrogelsCharacterizationX-ray crystallographyRheologyRheometryViscometryHeegerMacDiarmidShirakawa Edwardsde GennesZieglerStaudingerGoodyearBaekelandHaywardBraconnotExtrusionBlow moldingApplied coatingsProtective Coatings3D printingConsumer productsWhitewallsCookware and bakewareBakeliteFood ContainerVinyl recordKevlarPlastic bottlePlastic bagromanizedstereochemistrychiralmacromoleculepolymercrystallineamorphoussolublesolventtetrahedral molecular geometry,Natta projection after Giuliorepeating unitsubstituentbackbonestereoisomericmeso compoundBernoulli processcarbon-13 NMRZiegler–Natta catalysissemicrystallinemetallocene catalysis polymerizationmelting pointGutta perchafree-radical mechanisms, suchpolyvinyl chlorideprotoncarbon-13BernoullianMarkovian analysisx-ray powder diffractionsecondary ion mass spectrometryPure and Applied ChemistryWayback MachineBibcodeUniversity of California, Los Angeles