Anthranilic acid
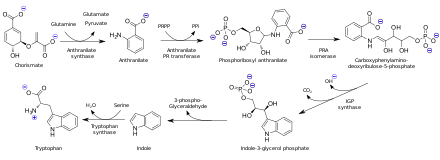
[10] Above 81 °C (178 °F; 354 K), it converts to an orthorhombic form with space group Pbca, which is not triboluminescent; a non-triboluminescent monoclinic phase with similar structure is also known.[10] In 1840-1841, Carl Julius Fritzsche was able to extract and crystallize two acids from the products of reaction of indigo dye with caustic potash, which he called chrysanilic and anthranilic acids after their colors before purification (golden yellow and black respectively) and the plant anil (Indigofera suffruticosa).This cation can be used to generate benzyne,[20] dimerized to give diphenic acid,[21] or undergo diazonium coupling reactions such as in the synthesis of methyl red.[23] Chlorination of anthranilic acid gives the 2,4-dichloro derivative, which can undergo reductive coupling to form a biaryl compound.[24] It is also a DEA List I Chemical because of its use in making the now-widely outlawed euphoric sedative drug methaqualone (Quaalude, Mandrax).

Preferred IUPAC nameCAS NumberBeilstein ReferenceChEMBLChemSpiderDrugBankECHA InfoCardEC NumberGmelin ReferencePubChemRTECS numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilitychloroformpyridineethanolethyl ethertrifluoroacetic acidbenzeneVapor pressureAcidityMagnetic susceptibilityRefractive indexStd enthalpy offormationGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointAutoignitiontemperatureSafety data sheetLegal statusstandard statearomatic acidformulaortho-substitutedcarboxylic acidfunctional groupsamphotericamino acidzwitterionicmonoclinictriboluminescentorthorhombicCarl Julius Fritzscheindigo dyecaustic potashIndigofera suffruticosaCahoursphthalic anhydrideHofmann rearrangementhypochloritephthalimidehypobromitechorismic acidanthranilate synthasetryptophanphosphoribosyl pyrophosphateamine groupindoleazo dyessaccharinestersperfumesjasmineorangeloop diureticsfurosemidecorrosion inhibitorsmold inhibitorssoy sauceAnthranilate-based insect repellentsFenamic acidisosteresalicylic acidactive metaboliteaspirinnon-steroidal anti-inflammatory drugsmefenamic acidtolfenamic acidflufenamic acidmeclofenamic aciddiazonium cationbenzynediphenic aciddiazonium couplingmethyl redphosgeneisatoic anhydrideChlorinationreductivebiarylDEA List I ChemicalmethaqualoneKynureninase3-Aminobenzoic acid4-Aminobenzoic acidMethyl anthranilateThe Royal Society of ChemistryCRC Handbook of Chemistry and PhysicsCRC PressAnvisaDiário Oficial da UniãoFlorida State UniversityBibcodeOrganic Synthesesmetabolismmetabolic intermediatesacetyl-CoAlysineSaccharopineAllysineα-Aminoadipic acid2-Oxoadipic acidGlutaryl-CoAGlutaconyl-CoACrotonyl-CoAβ-Hydroxybutyryl-CoA