Aldehyde
In their 1H NMR spectra, the formyl hydrogen center absorbs near δH 9.5 to 10, which is a distinctive part of the spectrum.The 13C NMR spectra of aldehydes and ketones gives a suppressed (weak) but distinctive signal at δC 190 to 205.Traces of many aldehydes are found in essential oils and often contribute to their pleasant odours, including cinnamaldehyde, cilantro, and vanillin.It involves treatment of the alkene with a mixture of hydrogen gas and carbon monoxide in the presence of a metal catalyst.Illustrative is the generation of butyraldehyde by hydroformylation of propylene: One complication with this process is the formation of isomers, such as isobutyraldehyde: The largest operations involve methanol and ethanol respectively to formaldehyde and acetaldehyde, which are produced on multimillion ton scale annually.Other large scale aldehydes are produced by autoxidation of hydrocarbons: benzaldehyde from toluene, acrolein from propylene, and methacrolein from isobutene.[2] From the industrial perspective, important reactions are: Because of resonance stabilization of the conjugate base, an α-hydrogen in an aldehyde is weakly acidic with a pKa near 17.[15] But it becomes the dominant tautomer in strong acid or base solutions, and enolized aldehydes undergo nucleophilic attack at the α position.The combination of manganese dioxide, cyanide, acetic acid and methanol will convert the aldehyde to a methyl ester.In the acetalisation reaction, under acidic or basic conditions, an alcohol adds to the carbonyl group and a proton is transferred to form a hemiacetal.Another aldehyde molecule can also act as the nucleophile to give polymeric or oligomeric acetals called paraldehydes.In alkylimino-de-oxo-bisubstitution, a primary or secondary amine adds to the carbonyl group and a proton is transferred from the nitrogen to the oxygen atom to create a carbinolamine.In the case of a primary amine, a water molecule can be eliminated from the carbinolamine intermediate to yield an imine or its trimer, a hexahydrotriazine This reaction is catalyzed by acid.An ammonia derivative of the form H2NNR2 such as hydrazine (H2NNH2) or 2,4-dinitrophenylhydrazine can also be the nucleophile and after the elimination of water, resulting in the formation of a hydrazone, which are usually orange crystalline solids.Some aldehydes are produced only on a small scale (less than 1000 tons per year) and are used as ingredients in flavours and perfumes such as Chanel No.The common names for aldehydes do not strictly follow official guidelines, such as those recommended by IUPAC, but these rules are useful.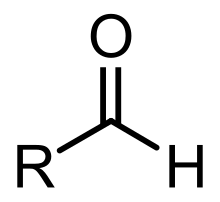
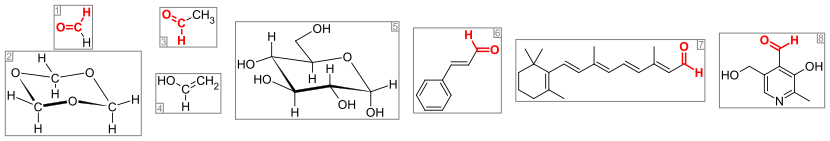
organic chemistryorganic compoundfunctional groupside chainhybridizedpicometersformaldehydeacetaldehydeIR spectroscopy1H NMR1,3,5-trioxanevinyl alcoholglucosecinnamaldehyderetinalopsinsphotoreceptorspyridoxalessential oilscilantrovanillinaldoseshemiacetalshydroformylationbutyraldehydepropylenemethanolethanolautoxidationbenzaldehydetolueneacroleinmethacroleinisobuteneWacker processoxidizing agentschromium(VI) reagents are popularpotassium dichromatedichromatecarboxylic aciddistilledvolatilehypervalent organoiodine compoundsIBX acidDess–Martin periodinanealso oxidize the α positionLux-Flood acidvarious sulfoxidesSwern oxidationGanem oxidationnitroxylscatalyze aldehyde formation with a cheaper oxidantvicinal diolsoxidized sequelaeacyloinsα-hydroxy acidscleavagecarbon dioxideOzonolysisAlkeneswork-upsinglet oxygenCarbonyl reductionEstersamidesDIBAL-Hsodium aluminium hydrideamide reductionRosenmund reactionAcyl chloridesreducedWittig reactionKetonesmethoxymethylenetriphenylphosphineFormylation reactionsNucleophilicarenesVilsmeier-Haack reactionNef reactionNitro compoundshydrolysisprimaryKornblum oxidationHaloalkanesdimethyl sulfoxideZincke reactionPyridinesZincke aldehydesStephen aldehyde synthesisNitrilesiminiumtin(II) chlorideGeminal halide hydrolysisGeminaldihalidesMeyers synthesisOxazinesHemiaminaloxalic acidHofmann rearrangementUnsaturatedhydroxyintermediatecarbamateMcFadyen-Stevens reactionHydrazidescatalyzedthermal decompositionBiotransformationLyophilizedTrametes hirsutaplasticizerspolyolsresonance stabilizationα-hydrogenacidicenolatetautomerKeto–enol tautomerismnucleophilic attack at the α positionAldehyde reductionprimary alcoholhydrogenationtransfer hydrogenationStoichiometricsodium borohydride