Atmosphere of Earth
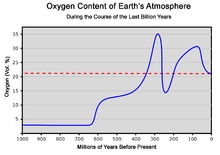
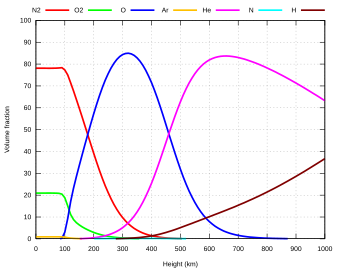
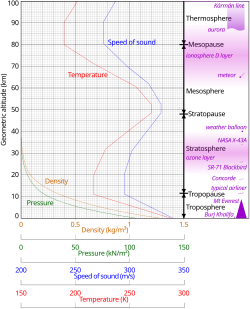
The atmosphere serves as a protective buffer between the Earth's surface and outer space, shields the surface from most meteoroids and ultraviolet solar radiation, keeps it warm and reduces diurnal temperature variation (temperature extremes between day and night) through heat retention (greenhouse effect), redistributes heat and moisture among different regions via air currents, and provides the chemical and climate conditions allowing life to exist and evolve on Earth.By mole fraction (i.e., by quantity of molecules), dry air contains 78.08% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other trace gases (see Composition below for more detail).Several layers can be distinguished in the atmosphere based on characteristics such as temperature and composition, namely the troposphere, stratosphere, mesosphere, thermosphere (formally the ionosphere) and exosphere.The concentration of water vapor (a greenhouse gas) varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5% by mole fraction in hot, humid air masses, and concentrations of other atmospheric gases are typically quoted in terms of dry air (without water vapor).Many substances of natural origin may be present in locally and seasonally variable small amounts as aerosols in an unfiltered air sample, including dust of mineral and organic composition, pollen and spores, sea spray, and volcanic ash.Because the general pattern of the temperature/altitude profile, or lapse rate, is constant and measurable by means of instrumented balloon soundings, the temperature behavior provides a useful metric to distinguish atmospheric layers.It extends from the thermopause (also known as the "exobase") at the top of the thermosphere to a poorly defined boundary with the solar wind and interplanetary medium.[18] This layer is mainly composed of extremely low densities of hydrogen, helium and several heavier molecules including nitrogen, oxygen and carbon dioxide closer to the exobase.[22][23] Just below the mesopause, the air is so cold that even the very scarce water vapor at this altitude can condense into polar-mesospheric noctilucent clouds of ice particles.These are the highest clouds in the atmosphere and may be visible to the naked eye if sunlight reflects off them about an hour or two after sunset or similarly before sunrise.This rise in temperature is caused by the absorption of ultraviolet radiation (UV) from the Sun by the ozone layer, which restricts turbulence and mixing.The troposphere is bounded above by the tropopause, a boundary marked in most places by a temperature inversion (i.e. a layer of relatively warm air above a colder one), and in others by a zone that is isothermal with height.However, the atmosphere is more accurately modeled with a customized equation for each layer that takes gradients of temperature, molecular composition, solar radiation and gravity into account.The combined absorption spectra of the gases in the atmosphere leave "windows" of low opacity, allowing the transmission of only certain bands of light.Systematic variations in the refractive index can lead to the bending of light rays over long optical paths.One example is that, under some circumstances, observers on board ships can see other vessels just over the horizon because light is refracted in the same direction as the curvature of Earth's surface.The first atmosphere, during the Early Earth's Hadean eon, consisted of gases in the solar nebula, primarily hydrogen, and probably simple hydrides such as those now found in the gas giants (Jupiter and Saturn), notably water vapor, methane and ammonia.During this earliest era, the Moon-forming collision and numerous impacts with large meteorites heated the atmosphere, driving off the most volatile gases.The collision with Theia, in particular, melted and ejected large portions of Earth's mantle and crust and outgassed significant amounts of steam which eventually cooled and condensed to contribute to ocean water at the end of the Hadean.[46]: 10 The increasing solidification of Earth's crust at the end of the Hadean closed off most of the advective heat transfer to the surface, causing the atmosphere to cool, which condensed most of the water vapor out of the air precipitating into a superocean.Ancient sediments in the Gabon dating from between about 2.15 and 2.08 billion years ago provide a record of Earth's dynamic oxygenation evolution.Before this time, any oxygen produced by cyanobacterial photosynthesis would be readily removed by the oxidation of reducing substances on the Earth's surface, notably ferrous iron, sulfur and atmospheric methane.O2 showed major variations during the Proterozoic, including a billion-year period of euxinia, until reaching a steady state of more than 15% by the end of the Precambrian.[52] The rise of the more robust eukaryotic photoautotrophs (green and red algae) injected further oxygenation into the air, especially after the end of the Cryogenian global glaciation, which was followed by an evolutionary radiation event during the Ediacaran period known as the Avalon explosion, where complex metazoan life forms (including the earliest cnidarians, placozoans and bilaterians) first proliferated.The following time span from 539 million years ago to the present day is the Phanerozoic eon, during the earliest period of which, the Cambrian, more actively moving metazoan life began to appear and rapidly diversify in another radiation event called the Cambrian explosion, whose locomotive metabolism was fuelled by the rising oxygen level.Air pollution is the introduction of airborne chemicals, particulate matter or biological materials that cause harm or discomfort to organisms.[53] The population growth, industrialization and motorization of human societies have significantly increased the amount of airborne pollutants in the Earth's atmosphere, causing noticeable problems such as smogs, acid rains and pollution-related diseases.The depletion of stratospheric ozone layer, which shields the surface from harmful ionizing ultraviolet radiations, is also caused by air pollution, chiefly from chlorofluorocarbons and other ozone-depleting substances.Since 1750, human activity, especially after the Industrial Revolution, has increased the concentrations of various greenhouse gases, most importantly carbon dioxide, methane and nitrous oxide.[54] It has raised concerns of man-made climate change, which can have significant environmental impacts such as sea level rise, ocean acidification, glacial retreat (which threatens water security), increasing extreme weather events and wildfires, ecological collapse and mass dying of wildlife.








Air (disambiguation)Air qualityBlue light is scatteredstratospheretroposphereplanetary surfaceoceansaerosolsparticulatesweathercloudsEarth's gravityouter spacemeteoroidssolar radiationdiurnal temperature variationgreenhouse effectair currentschemicalclimateevolvemole fractionmoleculesnitrogenoxygencarbon dioxidetrace gasesaccretedsolar nebulavolcanismimpact eventsweatheringevolution of lifephotoautotrophsclimate changedeforestationfossil fuelglobal warmingozone depletionacid depositionKármán lineatmospheric reentrymesospherethermosphereionosphereexosphereatmospheric pressurealtitudephotosynthesisterrestrial plantsrespirationterrestrial animalsatmospheric scienceclimatologyatmospheric physicsLéon Teisserenc de BortRichard AssmannpaleoclimatologyAtmospheric chemistrygreenhouse gasesnoble gasesheliumkryptonchemical compoundspollensporessea sprayvolcanic ashpollutantschlorinefluorinemercuryhydrogen sulfidesulfur dioxideCarbon dioxideMethaneWater vaporexperimental errorvolume fractionparts per millionincreasing in recent decadesmolecular weight mass fractionlapse rateballoon soundingsthermopausesolar windinterplanetary mediumradiation pressuregeocoronaescape into spaceballistictrajectoriesmagnetospheremeteorologicalaurorasartificial satellitesexobasetemperature in the usual senseaurora borealisaurora australisInternational Space StationAfterglowatmospheric entryspacecraftmesopausenoctilucent cloudstransient luminous eventsthundercloudsmeteorssounding rocketsrocket-powered aircrafttropopausestratopausepressure at sea levelozone layerultraviolet radiationnacreous cloudsjet-powered aircraftcloud typeshigh altitudeshorizonblue scatteredgeographic polesEquatortemperature inversion