Silicon tetraiodide
It is a tetrahedral molecule with Si-I bond lengths of 2.432(5) Å.[1] SiI4 is a precursor to silicon amides of the formula Si(NR2)4 (R = alkyl).This compound is produced by treating silicon-copper mixture with iodine:[3] It reacts quickly with water and moisture in the air.Of more academic interest is the reaction of silane with iodine vapour at 130 - 150 °C, as this produces a series of compounds ranging from iodosilane SiH3I to diiodosilane SiH2I2 and triiodosilane SiHI3 as well.[4] The last one can be readily distinguished from the similar carbon compound, iodoform which is a yellow solid at room temperature.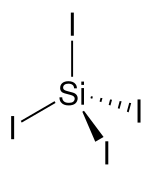



CAS NumberChemSpiderECHA InfoCardEC NumberPubChemCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilityorganic solventsMolecular shapetetrahedralGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704anionsSilicon tetrafluorideSilicon tetrachlorideSilicon tetrabromidecationsCarbon tetraiodideGermanium tetraiodideTin(IV) iodidestandard statechemical compoundmicroelectronicssilicon carbidesilaneiodosilaneiodoformGreenwood, Norman N.Butterworth-HeinemannAnswers.comSilicon compoundsSi2Cl6Si(N3)4Si2N2OSi2Cl6OSiF3ClsilicideSi4−Mn5Si3Fe5Si3more…iodideinorganiccompound