Potassium sodium tartrate
[3] This property led to its extensive use in crystal phonograph cartridges, microphones and earpieces during the post-World War II consumer electronics boom of the mid-20th century.It is then basified with hot saturated sodium hydroxide solution to pH 8, decolorized with activated charcoal, and chemically purified before being filtered.The salt is separated from the mother liquor by centrifugation, accompanied by washing of the granules, and is dried in a rotary furnace and sieved before packaging.[2] Larger crystals of Rochelle salt have been grown under conditions of reduced gravity and convection on board Skylab.[9] In 1919, Alexander McLean Nicolson worked with Rochelle salt, developing audio-related inventions like microphones and speakers at Bell Labs.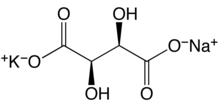


IUPAC nameCAS NumberChemSpiderECHA InfoCardEC NumberE numberPubChemCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilityCrystal structureCalcium tartrateMetatartaric acidPotassium antimonyl tartratePotassium tartrateSodium ammonium tartrateSodium tartratestandard statedouble salttartaric acidapothecaryLa RochelleFrancetartratemonopotassium phosphatepiezoelectricityphonograph cartridgestransducersdeliquescentlaxativesilveringFehling's solutionelectroplatingelectronicscigarette paperoxidizerpyrotechnicsemulsionshydridereagentprotein crystallographyBiuret reagentproteincupricSkylabtartarmother liquorsodium hydroxideactivated charcoalDavid BrewsterpiezoelectricpyroelectricityCRC Handbook of Chemistry and PhysicsUllmann's Encyclopedia of Industrial Chemistry