Potentiostat
A potentiostat is the electronic hardware required to control a three electrode cell and run most electroanalytical experiments.The heart of the different potentiostatic electronic circuits is an operational amplifier (op amp).This equipment is fundamental to modern electrochemical studies using three electrode systems for investigations of reaction mechanisms related to redox chemistry and other chemical phenomena.Modern potentiostats are designed to interface with a personal computer and operate through a dedicated software package.The automated software allows the user rapidly to shift between experiments and experimental conditions.The computer allows data to be stored and analyzed more effectively, rapidly, and accurately than the earlier standalone devices.As a result, the variable system resistance and the controlled current are inversely proportional Since 1942, when the English electrochemist Archie Hickling (University of Leicester) built the first three electrode potentiostat,[6] substantial progress has been made to improve the instrument.It adjusts its output to automatically control the cell current so that a condition of equilibrium is satisfied.Prior to observing the following equations, one may note that, from an electrical point of view, the electrochemical cell and the current measurement resistorAt this point the assumption may be made that a negligible amount of current is flowing through the reference electrode.This correlates to physical phenomenon since the reference electrode is connected to a high impedance electrometer.is the fraction of the output voltage of the control amplifier returned to its negative input; namely the feedback factor: Combining Eqs.The algorithm requires software-controllable hardware such as a digital multimeter, a power supply, and a double-pole double-throw relay.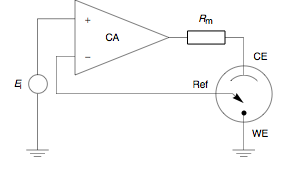

electronic hardwareelectroanalyticalpotentialworking electrodereference electrodecurrentauxiliary electrodeoperational amplifierelectric circuitop ampselectrochemicalthree electrode systemsreaction mechanismschemicalvoltammetryelectric currentelectric potentialvoltagebulk electrolysiscoulombselectric chargeamperespersonal computersoftwarecontrolmeasuringresistanceOhm's lawinversely proportionalelectrical resistanceUniversity of Leicestercounter electroderule of proportionsetpointdigital multimeterpower supplyanalyteultramicroelectrodesworking electrodesrotating ring-disk electroderotating disk electrodeAmperostatCoulometryElectroanalytical methodGalvanostatPolarographyPotentiometryHandbook of ElectrochemistryBibcodeFrontiers in Energy Researchvirtual instrumentation