Electric charge
Electric charge (symbol q, sometimes Q) is a physical property of matter that causes it to experience a force when placed in an electromagnetic field.Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects.Michael Faraday, in his electrolysis experiments, was the first to note the discrete nature of electric charge.Robert Millikan's oil drop experiment demonstrated this fact directly, and measured the elementary charge.The coulomb is defined as the quantity of charge that passes through the cross section of an electrical conductor carrying one ampere for one second.The Greeks observed that the charged amber buttons could attract light objects such as hair.[20] Other European pioneers were Robert Boyle, who in 1675 published the first book in English that was devoted solely to electrical phenomena.[21] His work was largely a repetition of Gilbert's studies, but he also identified several more "electrics",[22] and noted mutual attraction between two bodies.Further experiments (e.g., extending the cork by putting thin sticks into it) showed—for the first time—that electrical effluvia (as Gray called it) could be transmitted (conducted) over a distance.[23] Through these experiments, Gray discovered the importance of different materials, which facilitated or hindered the conduction of electrical effluvia.[23] Gray also discovered electrical induction (i.e., where charge could be transmitted from one object to another without any direct physical contact).[25] Gray's discoveries introduced an important shift in the historical development of knowledge about electric charge.The fact that electrical effluvia could be transferred from one object to another, opened the theoretical possibility that this property was not inseparably connected to the bodies that were electrified by rubbing.[26] In 1733 Charles François de Cisternay du Fay, inspired by Gray's work, made a series of experiments (reported in Mémoires de l'Académie Royale des Sciences), showing that more or less all substances could be 'electrified' by rubbing, except for metals and fluids[27] and proposed that electricity comes in two varieties that cancel each other, which he expressed in terms of a two-fluid theory.[30] Up until about 1745, the main explanation for electrical attraction and repulsion was the idea that electrified bodies gave off an effluvium.[31] Benjamin Franklin started electrical experiments in late 1746,[32] and by 1750 had developed a one-fluid theory of electricity, based on an experiment that showed that a rubbed glass received the same, but opposite, charge strength as the cloth used to rub the glass.In 1800 Alessandro Volta was the first to show that charge could be maintained in continuous motion through a closed path.[42] In 1833, Michael Faraday sought to remove any doubt that electricity is identical, regardless of the source by which it is produced.[45] In 1838, Faraday also put forth a theoretical explanation of electric force, while expressing neutrality about whether it originates from one, two, or no fluids.In developing a field theory approach to electrodynamics (starting in the mid-1850s), James Clerk Maxwell stops considering electric charge as a special substance that accumulates in objects, and starts to understand electric charge as a consequence of the transformation of energy in the field.[47] This pre-quantum understanding considered magnitude of electric charge to be a continuous quantity, even at the microscopic level.The exactly opposite properties of the two kinds of electrification justify our indicating them by opposite signs, but the application of the positive sign to one rather than to the other kind must be considered as a matter of arbitrary convention—just as it is a matter of convention in mathematical diagram to reckon positive distances towards the right hand.Beware that, in the common and important case of metallic wires, the direction of the conventional current is opposite to the drift velocity of the actual charge carriers; i.e., the electrons.This law is inherent to all processes known to physics and can be derived in a local form from gauge invariance of the wave function.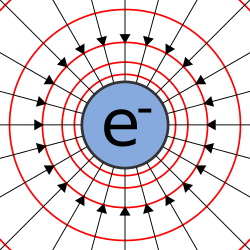

Electric fieldSI unitcoulombelementary chargefaradayampere-hourSI base unitsExtensiveConservedElectromagnetismElectricityMagnetismOpticsHistoryComputationalTextbooksPhenomenaElectrostaticsCharge densityConductorCoulomb lawElectretElectric dipoleElectric fluxElectric potentialElectrostatic dischargeElectrostatic inductionGauss lawInsulatorPermittivityPolarizationPotential energyStatic electricityTriboelectricityMagnetostaticsAmpère lawBiot–Savart lawGauss magnetic lawMagnetic dipoleMagnetic fieldMagnetic fluxMagnetic scalar potentialMagnetic vector potentialMagnetizationPermeabilityElectrodynamicsBremsstrahlungCyclotron radiationDisplacement currentEddy currentElectromagnetic fieldElectromagnetic inductionElectromagnetic pulseElectromagnetic radiationFaraday lawJefimenko equationsLarmor formulaLenz lawLiénard–Wiechert potentialLondon equationsLorentz forceMaxwell equationsMaxwell tensorPoynting vectorSynchrotron radiationElectrical networkAlternating currentCapacitanceCurrent densityDirect currentElectric currentElectric powerElectrolysisElectromotive forceImpedanceInductanceJoule heatingKirchhoff lawsNetwork analysisOhm lawResistanceVoltageWaveguidesMagnetic circuitAC motorDC motorElectric machineElectric motorGyrator–capacitorInduction motorLinear motorMagnetomotive forcePermeanceReluctance (complex)Reluctance (real)StatorTransformerCovariant formulationElectromagnetic tensorElectromagnetism and special relativityFour-currentFour-potentialMathematical descriptionsMaxwell equations in curved spacetimeRelativistic electromagnetismStress–energy tensorAmpèreEinsteinFizeauHeavisideHelmholtzHopkinsonJefimenkoKelvinKirchhoffLarmorLiénardLorentzMaxwellNeumannØrstedPoissonPoyntingRitchieSavartSinger