Imidazole
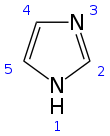
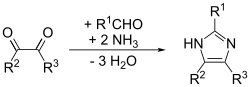
Imidazole (ImH) is an organic compound with the formula (CH)3(NH)N. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution.This ring system is present in important biological building blocks, such as histidine and the related hormone histamine.[11] Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric forms because hydrogen can be bound to one or another nitrogen atom.In one microwave modification, the reactants are benzil, benzaldehyde and ammonia in glacial acetic acid, forming 2,4,5-triphenylimidazole ("lophine").The example below applies to imidazole when R1 = R2 = hydrogen.The (1,2) and (2,3) bonds can be formed by treating a 1,2-diaminoalkane, at high temperatures, with an alcohol, aldehyde, or carboxylic acid.Imidazole is part of the theophylline molecule, found in tea leaves and coffee beans, that stimulates the central nervous system.[18][19] Other biological activities of the imidazole pharmacophore relate to the downregulation of intracellular Ca2+ and K+ fluxes, and interference with translation initiation.[24] Polyethylene terephthalate (PET) is a widely used plastic found in clothing, food packaging, beverage bottles, and thermoplastic resins.The massive accumulation of PET waste, mostly from single-use beverage bottles and food packaging, creates serious environmental problems.[25] As a type of polyester, PET can be broken down through a process called depolymerization which involves breaking its molecular chains using chemical methods.[26] TBI can be further processed into smaller products, including amides, benzimidazoles, and esters, or even reused to create new polymers.This may allow manufacturers to delay deciding on the final products until after the depolymerization process, providing flexibility to meet different industrial demands.Imidazole is used to elute tagged proteins bound to nickel ions attached to the surface of beads in the chromatography column.



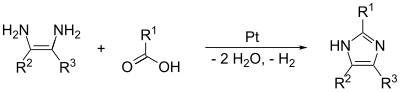




Preferred IUPAC nameCAS NumberBeilstein ReferenceChEMBLChemSpiderDrugBankECHA InfoCardEC NumberGmelin ReferencePubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterAcidityconjugate acidUV-visCrystal structureMonoclinicCoordination geometryOccupational safety and healthGHS labellingPictogramsHazard statementsPrecautionary statementsFlash pointstandard stateorganic compoundalkalineheterocyclediazolealkaloidshistidinehistamineantifungal drugsnitroimidazoleantibioticsmidazolampyrimidinepurineArthur Rudolf Hantzschtautomericnitrogenelectric dipole momentaromaticπ-electronsresonanceamphotericpyridineHeinrich Debusglyoxalformaldehydeammoniamicrowavebenzilbenzaldehydeglacial acetic acidlophineDebus methodfunctional groupsimidatealdehydeacetalalkanealcoholcarboxylic acidplatinumaluminaformamideDebus-Radziszewski imidazole synthesisphotolysis1-vinyltetrazoleorganotin compoundethylenediamineVan Leusen reactionTosMICaldimineamino acidside-chainproteinsenzymeshemoglobindecarboxylatedurticariaallergicmercaptopurinefungicidesantifungalantiprotozoalantihypertensivetheophyllinecentral nervous systemclotrimazolenitric oxide synthasepharmacophorefungalinfectionsketoconazolemiconazolefluconazoleitraconazolevoriconazolecytochrome P450 enzymesulconazole nitratekeratinAnthrenocerus australiseconazole nitrateTineola bisselliellaenilconazoleclimbazoleprochlorazbifonazoleagrichemicalsPolyethylene terephthalatedepolymerizationTransition metal imidazole complexHis-taggednickelchromatographyLowry protein assay