Fatty acid synthase
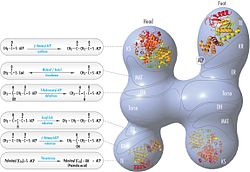
[1][6][7][8][9] Its main function is to catalyze the synthesis of palmitate (C16:0, a long-chain saturated fatty acid) from acetyl-CoA and malonyl-CoA, in the presence of NADPH.Following each round of elongation the beta keto group is reduced to the fully saturated carbon chain by the sequential action of a ketoreductase (KR), dehydratase (DH), and enoyl reductase (ER).[10] Mammalian FAS consists of a homodimer of two identical protein subunits, in which three catalytic domains in the N-terminal section (-ketoacyl synthase (KS), malonyl/acetyltransferase (MAT), and dehydrase (DH)), are separated by a core region (known as the interdomain) of 600 residues from four C-terminal domains (enoyl reductase (ER), -ketoacyl reductase (KR), acyl carrier protein (ACP) and thioesterase (TE)).However, in both cases the conserved ACP acts as the mobile domain responsible for shuttling the intermediate fatty acid substrates to various catalytic sites.A first direct structural insight into this substrate shuttling mechanism was obtained by cryo-EM analysis, where ACP is observed bound to the various catalytic domains in the barrel-shaped yeast fatty acid synthase.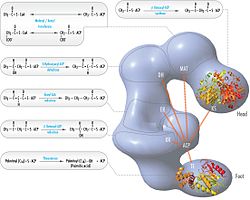
EC no.CAS no.IntEnzBRENDAExPASyMetaCycGene OntologyPubMedAliasesGeneCardsOrthologsEntrezEnsemblUniProtWikidataenzymeproteinfatty acid synthesispolypeptidessubstratespalmitatesaturated fatty acidacetyl-CoAmalonyl-CoAClaisen condensationketoreductasedehydrataseenoyl reductaseacyl carrier proteinthioesteraseanimalscorynebacteriamycobacterianocardiaantibioticspolyketide synthasescatalyticN-terminalC-terminalcysteinephosphopantetheineheterodimericMetabolismhomeostasissterol regulatory element binding proteinliver X receptorsphloroglucinolsDryopteris crassirhizomaoncogeneupregulatedinhibitorsdrug discoveryligandPPARalphafibrateOrlistatestrogen receptor alphaN-terminusC-terminusuterine leiomyomataDiscovery and development of gastrointestinal lipase inhibitorsFatty acid metabolismFatty acid degradationEnoyl-acyl carrier protein reductaseList of fatty acid metabolism disordersBibcodeMedical Subject HeadingsWayback MachineEnzymesmultienzyme complexesPhotosynthesisPhotosynthetic reaction center complex proteinsPhotosystemDehydrogenasePyruvate dehydrogenase complexOxoglutarate dehydrogenaseBranched-chain alpha-keto acid dehydrogenase complexBCKDHABCKDHBCarbamoyl phosphate synthase IIAspartate carbamoyltransferaseDihydroorotaseP450-containing systemsCytochrome b6f complexElectron transport chainGlycine decarboxylase complexMitochondrial trifunctional proteinPhosphoenolpyruvate sugar phosphotransferase systemPolyketide synthaseSucrase-isomaltase complexTryptophan synthaselipid metabolismtriglyceridefatty acidATP citrate lyaseAcetyl-CoA carboxylaseBeta-ketoacyl-ACP synthaseΒ-Ketoacyl ACP reductase3-Hydroxyacyl ACP dehydraseEnoyl ACP reductasedesaturasesStearoyl-CoA desaturase-1Glycerol-3-phosphate dehydrogenaseThiokinaseDegradationCarnitine palmitoyltransferase ICarnitine-acylcarnitine translocaseCarnitine palmitoyltransferase IIBeta oxidationAcyl CoA dehydrogenaseACADVLACADSBEnoyl-CoA hydrataseAcetyl-CoA C-acyltransferaseEnoyl CoA isomerase2,4 Dienoyl-CoA reductasePropionyl-CoA carboxylaseHydroxyacyl-Coenzyme A dehydrogenaseMalonyl-CoA decarboxylaseAldehydesLong-chain-aldehyde dehydrogenaseTransferasesacyltransferasesacetyltransferasesAcetyl-Coenzyme A acetyltransferaseN-Acetylglutamate synthase