tert-Butyl chloride
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl.It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol.It is produced industrially as a precursor to other organic compounds.[2] The overall reaction, therefore, is: Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.When dissolved in alcohols, the corresponding t-butyl ethers are produced.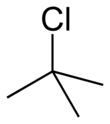


Preferred IUPAC nameCAS NumberChEMBLChemSpiderECHA InfoCardEC NumberPubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterVapor pressureGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointAutoignitiontemperaturealkyl halidestert-Butyl bromidestandard stateorganochloridehydrolysishydrogen chloridehydrochloric acidleaving groupcarbocationtert-butyl alcoholIsobutane