Strictosidine
It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase.Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids.[1][2] Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.Recent efforts in metabolic engineering have permitted the synthesis of strictosidine by yeast (Saccharomyces cerevisiae).The involvement of the glucoalkaloid strictosidine in the antimicrobial and antifeedant activity of Catharanthus roseus leaves was studied.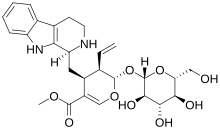
IUPAC nameCAS NumberChemSpiderPubChemCompTox DashboardSMILESChemical formulaMolar massMelting pointstandard statechemical compoundvinca alkaloidPictet–Spenglercondensation reactiontryptaminesecologaninstrictosidine synthasederivativesmonoterpeneindole alkaloidsbiosynthesisquininecamptothecinajmalicineserpentinevinblastinevincristinemitragynineApocynaceaeRhazya strictaCatharanthus roseusLoganiaceaeRubiaceaeIcacinaceaeNyssaceaeAlangiaceaeSaccharomyces cerevisiaeBibcode