Inverted sugar syrup
Invertase is added at a rate of about 0.15% of the syrup's weight, and inversion time will be about 8 hours.[4] Though inverted sugar syrup can be made by heating table sugar in water alone, the reaction can be sped up by adding lemon juice, cream of tartar, or other catalysts, often without changing the flavor noticeably.[citation needed] Common sugar can be inverted quickly by mixing sugar and citric acid or cream of tartar at a ratio of about 1000:1 by weight and adding water.If lemon juice, which is about five percent citric acid by weight, is used instead then the ratio becomes 50:1.Such a mixture, heated to 114 °C (237 °F)[5] and added to another food, prevents crystallization without tasting sour.Commercially prepared hydrochloric-acid catalyzed solutions may be inverted at the relatively low temperature of 50 °C (122 °F).[6][7] In confectionery and candy making, cream of tartar is commonly used as the acidulant, with typical amounts in the range of 0.15–0.25% of the sugar's weight.Syrup is used to feed microbiological life, which requires oxygen found in the water.For example, kombucha is produced by fermenting inverted sugar syrup with tea using a symbiotic culture of bacteria and yeast (SCOBY), and yeast in winemaking is used for ethanol fermentation.Water in a container with wide bottom surface area allows for faster dissolving of the sucrose, which only has to be mixed a few times periodically to form a homogeneous solution.Heating a mixture or solution of table sugar and water breaks the chemical bond that links together the two simple-sugar components.Plane polarized light can be shone through a sucrose solution as it is heated up for hydrolysis.When such light is shone through a solution of pure sucrose it comes out the other side with a different angle than when it entered, which is proportional to both the concentration of the sugar and the length of the path of light through the solution; its angle is therefore said to be 'rotated' and how many degrees the angle has changed (the degree of its rotation or its 'optical rotation') is given a letter name,When plane polarized light enters and exits a solution of pure sucrose its angle is rotated 66.5° (clockwise or to the right).As the sucrose is heated up and hydrolyzed the amount of glucose and fructose in the mixture increases and the optical rotation decreases.This leads to the definition of an 'inversion point' as the per cent amount sucrose that has to be hydrolyzed beforeAny solution which has passed the inversion point (and therefore has a negative value ofAs the shapes of the molecules ('chemical structures') of sucrose, glucose, and fructose are all asymmetrical the three sugars come in several different forms, called stereoisomers.When plane polarized light passes through a pure solution of one of these forms of one of the sugars it is thought to hit and 'glance off' certain asymmetrical chemical bonds within the molecule of that form of that sugar.Because those particular bonds (which in cyclic sugars like sucrose, glucose, and fructose include an anomeric bond) are different in each form of the sugar, each form rotates the light to a different degree.In the circumstance of 20 °C, the specific optical rotation of sucrose is known to be 66.6°, glucose is 52.2°, and fructose is −92.4°.[13] Water molecules do not have chirality, therefore they do not have any effect on the measurement of optical rotation.When plane polarized light enters a body of pure water its angle is no different from when it exits.Chemicals that, like water, have specific rotations that equal zero degrees are called 'optically inactive' chemicals and like water, they do not need to be considered when calculating optical rotation, outside of the concentration and path length.is a number used to identify the chemical species); and if each species has a specific rotation (the optical rotation of that chemical were it made as a pure solution) written asAssuming no extra chemical products are formed by accident (that is, there are no side reactions) a completely hydrolyzed sucrose solution no longer has any sucrose and is a half-and-half mixture of glucose and fructose.If a sucrose solution has been partly hydrolyzed, then it contains sucrose, glucose and fructose and its optical rotation angle depends on the relative amounts of each for the solution;do not need to be known to make use of this equation as the inversion point (per cent amount of sucrose that must be hydrolyzed before the solution is inverted) can be calculated from the specific rotation angles of the pure sugars.It can be shown that the solution's optical rotation angle is a function of (explicitly depends on) this per cent reaction progress.A polarimeter can be used to figure out when the inversion is done by detecting whether the optical rotation of the solution at an earlier time in its hydrolysis reaction equals −12.7°.



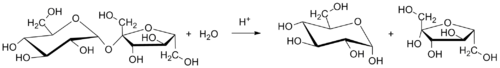
CAS NumberChEMBLChemSpiderPubChemMolar massATC codeC05BB03standard statemixturemonosaccharidesglucosefructosehydrolyticsaccharificationdisaccharidesucroseoptical rotationsweetertable sugarBakerssweetenersenzymeInvertasereactionlemon juicecream of tartarcatalystscitric acidhydrochloric-acidbaking sodafermentationkombuchasymbioticyeast in winemakingethanol fermentationsurface areablenderFree sugarCadbury Creme EggsfondantGolden syrupcofactorenrobedSour Patch Kidssweetened beveragesSweet reserveunfermentedCandi sugardubbeltripelhydrolysissolutionchemical bondbalanced chemical equationchemical propertypolarimetershapes of the moleculesasymmetricalstereoisomerschemical bondscyclic sugarsanomeric bond'averaging' effectchiralitychemical speciesmolar concentrationmole fractionside reactionsstoichiometryreaction progressfunctionHigh-fructose corn syrupList of syrupsGoogle BooksBibcodeList of sugars and sugar productsMonosaccharideGalactoseXyloseLactoseMaltoseTrehaloseAdded sugarReducing sugarSugar beetSugarcaneAgave syrupCoconutHoneydewSyrupsBarley malt syrupBrown rice syrupCheongMaesil-cheongMogwa-cheongYuja-cheongCorn syrupHigh-fructoseHigh-maltoseGlucose syrupKuromitsuMaple syrupMizuameMolassesPine honeySteen's cane syrupTable syrupTreacleYacón syrupPeen tongChancacaCrystalline fructoseGellingGula melakaJaggeryMolasses sugarMuscovadoNon-centrifugal cane sugarPanelaPlantation ReservePowderedPreservingSucanatSugar candyBarley sugarButterscotch