Proton-exchange membrane fuel cell
The proton-exchange membrane is commonly made of materials such as perfluorosulfonic acid (PFSA, sold commercially as Nafion and Aquivion), which minimize gas crossover and short circuiting of the fuel cell.Low Operating Temperature Under extreme sub-freezing conditions, the water produced by fuel cells can freeze in porous layers and flow channels.This new design enabled the first FC stack functions without a humidifying system meanwhile overcoming water recirculation issues and achieving high power output stability[54].Due to this repeating micro-scale convective flow, oxygen transport to catalyst layer (CL) and liquid water removal from GDL is significantly enhanced.The carbon support functions as an electrical conductor; the Pt particles are reaction sites; the ionomer provides paths for proton conduction, and the Teflon binder increases the hydrophobicity of the electrode to minimize potential flooding.[citation needed] Main factors that create losses are: The external electrodes, often referred to as bipolar plates or backplates, serve to distribute fuel and oxygen uniformly to the catalysts, to remove water, to collect and transmit electric current.Titanium nitride (TiN) is a cheaper material that is used in fuel cell backplates due to its high chemical stability, electrical conductivity, and corrosion resistance.Metal-organic frameworks (MOFs) are a relatively new class of porous, highly crystalline materials that consist of metal nodes connected by organic linkers.MOFs exhibit many unique properties due to their tunable pore sizes, thermal stability, high volume capacities, large surface areas, and desirable electrochemical characteristics.On the other hand, pores can be filled with additional ion carriers that ultimately enhance the ionic conductivity of the system and high crystallinity makes the design process less complex.[27] While polymeric materials are currently the preferred choice of proton-conducting membrane, they require humidification for adequate performance and can sometimes physically degrade due to hydrations effects, thereby causing losses of efficiency.As mentioned, Nafion is also limited by a dehydration temperature of < 100 °C, which can lead to slower reaction kinetics, poor cost efficiency, and CO poisoning of Pt electrode catalysts.A low temperature example is work by Kitagawa, et al. who used a two-dimensional oxalate-bridged anionic layer framework as the host and introduced ammonium cations and adipic acid molecules into the pores to increase proton concentration.However, this model holds promise for its temperature regime, anhydrous conditions, and ability to control the quantity of guest molecules within the pores, all of which allowed for the tunability of proton conductivity.Additionally, the triazole-loaded PCMOF2 was incorporated into a H2/air membrane-electrode assembly and achieved an open circuit voltage of 1.18 V at 100 °C that was stable for 72 hours and managed to remain gas tight throughout testing.Once MOFs are able to consistently achieve sufficient conductivity levels, mechanical strength, water stability, and simple processing, they have the potential to play an important role in PEMFCs in the near future.The high volumetric density, large pore surface areas, and openness of metal-ion sites in MOFs make them ideal candidates for catalyst precursors.The U.S. Department of Energy estimates that platinum-based catalysts will need to use roughly four times less platinum than is used in current PEM fuel cell designs in order to represent a realistic alternative to internal combustion engines.[32] Consequently, one main goal of catalyst design for PEM fuel cells is to increase the catalytic activity of platinum by a factor of four so that only one-fourth as much of the precious metal is necessary to achieve similar performance.Decreasing the particles' size alone increases the total surface area of catalyst available to participate in reactions per volume of platinum used, but recent studies have demonstrated additional ways to make further improvements to catalytic performance.[37][38] Very recently, a new class of ORR electrocatalysts have been introduced in the case of Pt-M (M-Fe[39] and Co) systems with an ordered intermetallic core encapsulated within a Pt-rich shell.While the observed enhancement in the activities is ascribed to a strained lattice, the authors report that their findings on the degradation kinetics establish that the extended catalytic durability is attributable to a sustained atomic order.[43] The mechanism that produces this effect is conceptually similar to that described for Pt3Ni above: the ruthenium core of the particle alters the electronic structure of the platinum surface, rendering it better able to catalyze the oxidation of CO.[44] Although the Pt loading of PEM fuel cells has been reduced by two orders of magnitude over the past decade,[45] further reduction is necessary to make the technology economically viable for commercialization.The U.S. Department of Energy has been setting milestones for the development of fuel cells, targeting a durability of 5000 hours and a non-PGM catalyst ORR volumetric activity of 300 A cm−3.[48][49] The major application of PEM fuel cells focuses on transportation primarily because of their potential impact on the environment, e.g. the control of emission of the green house gases (GHG).There is potential for PEMFCs to be used for stationary power generation, where they provide 5 kW at 30% efficiency; however, they run into competition with other types of fuel cells, mainly SOFCs and MCFCs.In the late 1980s and early 1990s, Los Alamos National Lab and Texas A&M University experimented with ways to reduce the amount of platinum required for PEM cells.[53] Parallel with Pratt and Whitney Aircraft, General Electric developed the first proton exchange membrane fuel cells (PEMFCs) for the Gemini space missions in the early 1960s.Several pivotal innovations, such as low platinum catalyst loading and thin film electrodes, drove the cost of fuel cells down, making development of PEMFC systems more realistic.

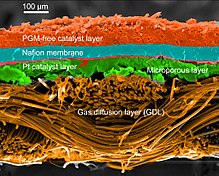

polymer electrolyte membranefuel cellstationary fuel-cell applicationsPEM electrolysisalkaline fuel-cellSpace Shuttlemembrane electrode assemblieschemical energyelectrochemicalelectrical energycombustionthermal energycatalyticallyprotonselectronsoxidation half-cell reactionload circuitcathodecurrentreductionoxygen reduction reactionstandard hydrogen electrodeshort circuitmoleculeplatinumperfluorosulfonic acidNafionelectroosmotic pumpwater gas shift reactionmethanolrutheniumparts per millionbiofuelsChemourspolybenzimidazole (PBI)phosphoric acidHigh Temperature Proton Exchange Membrane fuel cellsulfuric acidGibbs free energyheating valueU.S. Department of Energyinternal combustion enginesnanoparticlesMiller indexeselectronic structureadsorptionUltrasonic nozzlecarbon paperinkjet printingelectrolysissteam reforminghydrocarbons(100) facetssulfatefossil fuelssolid-oxide fuel cellsGeneral ElectricionomerGeminiAlkaline fuel cellsApolloPratt and WhitneyGemini space missionsGemini VApollo space missionsApollo-SoyuzSkylabcatalystautomobilesvehicleshydrogen economyToyota MiraiDynamic hydrogen electrodeGas diffusion electrodeGlossary of fuel cell termsHydrogen sulfide sensorPower-to-weight ratioReversible hydrogen electrodeTimeline of hydrogen technologiesBibcodeJournal of Materials ChemistryEnergy & Environmental ScienceJournal of Materials Chemistry AInternational Journal of Energy ResearchWayback MachineRoyal Society of ChemistryFuel cellsAlkaline fuel cellMolten carbonate fuel cellPhosphoric acid fuel cellSolid oxide fuel cellDirect borohydride fuel cellDirect carbon fuel cellDirect-ethanol fuel cellDirect methanol fuel cellFormic acid fuel cellMetal hydride fuel cellReformed methanol fuel cellZinc–air batteryEnzymatic biofuel cellMicrobial fuel cellBlue energyElectro-galvanic fuel cellFlow batteryMembrane electrode assemblyMembraneless Fuel CellsPhotoelectrochemical cellProton-exchange membraneProtonic ceramic fuel cellRegenerative fuel cellSolid oxide electrolyzer cellUnitized regenerative fuel cellHydrogenEconomyStationStorageVehicleGlossary