Pentacarbon dioxide
Pentacarbon dioxide, officially penta-1,2,3,4-tetraene-1,5-dione, is an oxide of carbon (an oxocarbon) with formula C5O2 or O=C=C=C=C=C=O.The compound was described in 1988 by Günter Maier and others, who obtained it by pyrolysis of 2,4,6-tris(diazo)cyclohexane-1,3,5-trione (C6N6O3).[1][2]: 97 Diazo transfer can produce the latter compound from phloroglucinol.[1] It is stable at room temperature in solution.[1] The pure compound is stable up to −90 °C, at which point it polymerizes.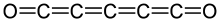

IUPAC nameCAS NumberChemSpiderPubChemCompTox DashboardSMILESChemical formulaMolar massstandard statecarbonoxocarbonpyrolysisDiazo transferphloroglucinolEthylene dioneCarbon suboxideOxocarbons1,2-Dioxetanedione1,3-DioxetanedioneCyclohexanehexoneEthylenetetracarboxylic dianhydrideC10O10C12O12Graphite oxideMetal carbonylsCarbonic acidBicarbonatesCarbonatesPolycarbonatesDicarbonatesTricarbonatesPeroxydicarbonates