Nitroethane
[5] Alternatively, nitroethane can be produced by the Victor Meyer reaction of haloethanes such as chloroethane, bromoethane, or iodoethane with silver nitrite in diethyl ether or THF.Nitroethane was previously used successfully as a chemical feedstock (precursor ingredient) in laboratories for the synthesis of multitudes of substances and consumer goods.For example, the medicine Pervitin (methamphetamine) was commonly used in the 19th and 20th century, and was especially popular during WWII by troops of both sides for mood elevation, appetite and sleep suppression and increasing focus and alertness).Nitroalkanes were one of many ingredients used in the synthesis of many phenethylamines, including medications such as Pervitin and the racemic compound Benzedrine[7] (amphetamine), used as an anorectic medicine for obesity.In animal studies, nitroethane exposure was observed to cause lacrimation, dyspnea, pulmonary rales, edema, liver and kidney injury, and narcosis.
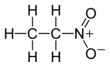


Preferred IUPAC nameCAS NumberChEMBLChemSpiderECHA InfoCardPubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterVapor pressureAcidityMagnetic susceptibilityViscosityOccupational safety and healthGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointExplosive limitsSafety data sheetnitro compounds2-NitropropaneNitromethaneEthyl nitriteEthyl nitratestandard stateorganic compoundpropanenitric acidexothermic1-nitropropaneVictor Meyerchloroethanebromoethaneiodoethanediethyl ethersodium nitritedimethyl sulfoxidedimethylformamideHenry reaction3,4-dimethoxybenzaldehydeantihypertensivemethyldopaphenyl-2-nitropropeneformaldehydehydrogenationsurfactantfuel additiverocket propellantspolystyrenecyanoacrylateartificial nailPervitinphenethylaminesracemicBenzedrineanorecticnervous systemdermatitislacrimationdyspneaNational Institute for Occupational Safety and Health