Sodium–potassium pump
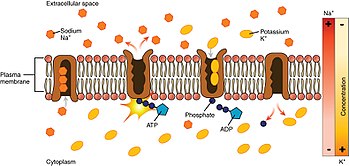
[1] The sodium–potassium pump was discovered in 1957 by the Danish scientist Jens Christian Skou, who was awarded a Nobel Prize for his work in 1997.[3] It also functions as a signal transducer/integrator to regulate the MAPK pathway, reactive oxygen species (ROS), as well as intracellular calcium.For instance, a study investigated the function of Na+/K+-ATPase in foot muscle and hepatopancreas in land snail Otala lactea by comparing the active and estivating states.The downstream signals through ouabain-triggered protein phosphorylation events include activation of the mitogen-activated protein kinase (MAPK) signal cascades, mitochondrial reactive oxygen species (ROS) production, as well as activation of phospholipase C (PLC) and inositol triphosphate (IP3) receptor (IP3R) in different intracellular compartments.For example, the Na+-K+ pump interacts directly with Src, a non-receptor tyrosine kinase, to form a signaling receptor complex.Based on this scenario, NaKtide, a peptide Src inhibitor derived from the Na+-K+ pump, was developed as a functional ouabain–Na+-K+ pump-mediated signal transduction.[20] This suggests that the pump might not simply be a homeostatic, "housekeeping" molecule for ionic gradients, but could be a computation element in the cerebellum and the brain.Note: Early studies indicated the opposite effect, but these were later found to be inaccurate due to additional complicating factors.[citation needed] The Na+/K+-ATPase is endogenously negatively regulated by the inositol pyrophosphate 5-InsP7, an intracellular signaling molecule generated by IP6K1, which relieves an autoinhibitory domain of PI3K p85α to drive endocytosis and degradation.In the case of patients where the heart is not pumping hard enough to provide what is needed for the body, use of digoxin helps to temporarily overcome this.Na+/K+-ATPase was proposed by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark.[39] Several studies have detailed the evolution of cardiotonic steroid resistance of the alpha-subunit gene family of Na/K-ATPase (ATP1A) in vertebrates via amino acid substitutions most often located in the first extracellular loop domain.[40][41][42][43][44][45][46] Amino acid substitutions conferring cardiotonic steroid resistance have evolved independently many times in all major groups of tetrapods.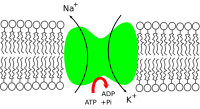


lipid bilayerEC no.IntEnzBRENDAExPASyMetaCycPubMedsodiumpotassiumadenosinetriphosphataseenzymeelectrogenictransmembraneATPasemembraneanimalcell physiologyactiveJens Christian Skounerve cellsP-type ATPasesresting potentialvolumeMAPK pathwayreactive oxygen speciesglycolysiscardiac Purkinje cellsglycogenintracellularintracellular spaceNernst potentialmembrane transport proteinsglucoseamino acidsinterstitial fluidsymporterrenal tubular systemosmolarityproteinsosmosisouabainprotein tyrosine phosphorylationmitogen-activated protein kinasephospholipase Cinositol triphosphatenon-receptor tyrosine kinaseankyrinPLCgamma1cofilincerebellarPurkinje neuronsaccessory olfactory bulbhomeostaticcomputationcerebellummutationdystoniaparkinsonismataxiaAlcoholaxolemmahydrolyzedphosphorylationaspartatedephosphorylationtriiodothyroninethyroidcardiac glycosidesdigoxininotropicsarcoplasmic reticulummusclecaveolaeEGF receptorcardiac glycosideUniversity of AarhusDenmarkNobel Prize in ChemistryATP1A1ATP1A2ATP1A3ATP1A4ATP1B1ATP1B3Drosophila melanogasterneofunctionalizationcardenolidesbufadienolidesSodium-calcium exchangerThyroid hormoneV-ATPaseBibcodeMedical Subject HeadingsKhan AcademyMembrane transport proteinion pumpsATPasesATP synthaseF-, V-, and A-type ATPase (3.A.2)H+ (F-type)ATP5A1ATP5C1ATP5F1ATP5MC1ATP5G2ATP5G3ATP5J2H+ (V-type)H+ transporting, lysosomalATP6AP1ATP6AP2ATP6V1AATP6V1B1ATP6V1B2ATP6V1C1ATP6V1C2ATP6V1DATP6V1E1ATP6V1E2ATP6V1FATP6V1G1ATP6V1G2ATP6V1G3ATP6V1HATP6V0A1