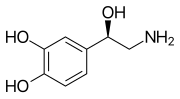
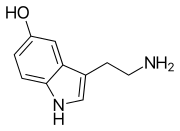
Monoamine neurotransmitter
They are deactivated in the body by the enzymes known as monoamine oxidases which clip off the amine group.Monoaminergic systems, i.e., the networks of neurons that use monoamine neurotransmitters, are involved in the regulation of processes such as emotion, arousal, and certain types of memory.[1] Drugs used to increase or reduce the effect of monoamine neurotransmitters are used to treat patients with psychiatric and neurological disorders, including depression, anxiety, schizophrenia and Parkinson's disease.[citation needed] After release into the synaptic cleft, monoamine neurotransmitter action is ended by reuptake into the presynaptic terminal.[12][13] A recent computational investigation of genetic origins shows that the earliest development of monoamines occurred 650 million years ago and that the appearance of these chemicals, necessary for active or participatory awareness and engagement with the environment, coincides with the emergence of bilaterian or “mirror” body in the midst of (or perhaps in some sense catalytic of?)
DopamineNorepinephrineSerotoninneurotransmittersneuromodulatorsaromatic ringamino acidsphenylalaninetyrosinetryptophanaromatic amino acid decarboxylaseenzymesmonoamine oxidasesneurotrophin-3depressionanxietyschizophreniaParkinson's diseasecatecholaminestrace amineshuman brainL-PhenylalanineL-TyrosineL-DOPAEpinephrinePhenethylaminep-TyramineN-MethylphenethylamineN-Methyltyraminep-OctopamineSynephrine3-MethoxytyramineCYP2D6Trace amineImidazoleaminesHistamineAdrenalineNoradrenalineIndolaminesMelatoninPhenethylaminesamphetaminePhenylethanolaminem-Tyraminem-OctopamineTryptamineproteinsmonoamine transportersdopamine transporterserotonin transporternorepinephrine transportercell membranevesicular monoamine transportervesiclessynaptic cleftenzymemonoamine oxidasemonoamine oxidase inhibitorsantidepressantsMonoamine reuptake inhibitorMonoamine receptorMonoamine transporterMonoamine HypothesisBiogenic amineMonoamine nucleiBiology of depressionBibcodeMedical Subject HeadingsAmino acidAgmatineAspartic acid (aspartate)Glutamic acid (glutamate)GlutathioneGlycineKynurenic acidProlineSerineα-Alanineβ-AlanineHypotaurineSarcosineTaurineGHB systemT-HCA (GHC)Biogenic aminesEpinephrine (adrenaline)NAS (normelatonin)Norepinephrine (noradrenaline)Serotonin (5-HT)3-IodothyronamineN-MethyltryptamineNeuropeptides2-AGE (noladin ether)AA-5-HTAnandamide (AEA)OleamideRVD-HpαVirodhamine (O-AEA)NeurosteroidsNucleobaseAdenosineVitaminAcetylcholineCarbon monoxide (CO)Hydrogen sulfide (H2S)Nitric oxide (NO)AcetaldehydeAmmonia (NH3)Carbonyl sulfide (COS)Nitrous oxide (N2O)Sulfur dioxide (SO2)Trace amine-associated receptormodulatorsAgonistsEndogenousβ-PhenethylamineCyclohexylamineTrimethylamineExogenous2C-B-Fly2C-T-7A-776362-AminoindaneApomorphineAsenapineBromocriptine