Adipocyte
If a child or adolescent gains sufficient excess weight, fat cells may increase in absolute number until age twenty-four.[4][5][6][7] Exercise reduces both adipocyte size as well as marrow adipose tissue volume, as quantified by MRI or μCT imaging of bone stained with the lipid binder osmium.Studies have shed light into potential molecular mechanisms in the fate determination of pre-adipocytes although the exact lineage of adipocyte is still unclear.[8][9] The variation of body fat distribution resulting from normal growth is influenced by nutritional and hormonal status dependent on intrinsic differences in cells found in each adipose depot.Reintroduction of an ordinary chow diet[13] to such animals precipitated a period of weight loss during which only mean adipocyte size returned to normal.[16] Approximately 10% of fat cells are renewed annually at all adult ages and levels of body mass index without a significant increase in the overall number of adipocytes in adulthood.[15] Obesity is characterized by the expansion of fat mass, through adipocyte size increase (hypertrophy) and, to a lesser extent, cell proliferation (hyperplasia).[17][2] In the fatty tissue of obese individuals, there is increased production of metabolism modulators, such as glycerol, hormones, macrophage-stimulating chemokines, and pro-inflammatory cytokines, leading to the development of insulin resistance.When sterol levels are depleted, INSIG1 releases SCAP and the SREBF1-SCAP complex can be sorted into transport vesicles coated by the coatomer COPII that are exported to the Golgi apparatus.This seems to help compensate for the anti-lipogenic effects of insulin resistance and thus preserve adipocyte fat storage abilities and availability of appropriate levels of fatty acid unsaturation in face of the nutritional pressures of obesity.
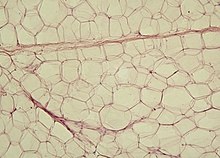

Anatomical terms of microanatomyadipose tissuemesenchymal stem cellsadipogenesiscell cultureosteoblastsmyocyteswhite adipose tissuebrown adipose tissuelipid dropletcytoplasmnucleustriglyceridescholesteryl esteradipokinesresistinadiponectinleptinapelinBrown fat cellspolyhedralwhite fatmitochondriamarrow adipose tissueμCT imagingosmiumLipoblasthistologyH&E stainfibroblastsconnective tissuelipoblastomaObesityhypertrophyhyperplasiafatty tissueglycerolhormonesmacrophagechemokinespro-inflammatory cytokinesinsulin resistancepathogenesisimmune systeminsulinpyruvate dehydrogenaseacetyl-CoA carboxylasefatty acid synthesisglucose uptakeSREBF1lipogenesissteroltranscription factorprecursor proteinendoplasmic reticulum (ER)helicesINSIG1vesiclescoatomerGolgi apparatustranslocatephospholipidsestrogensandrogensunderweightoverweightinfertilityList of human cell types derived from the germ layersBibcodeKahn SESoft tissueFibrosisScarringFibroblastFibrocyteReticular cellTendon cellMelanocyteWandering cellsMast cellExtracellular matrixGround substanceTissue fluidCollagen fibersReticular fibersCOL3A1Elastic fibersElastinFibrillinEMILIN1ElauninProperReticularAdiposeDense irregular connective tissueSubmucosaDermisDense regular connective tissueLigamentTendonAponeurosisMucoidMesenchymalCartilage
