Diisobutylaluminium hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2).A variety of techniques, not including X-ray crystallography, suggest that the compound exists as a dimer and a trimer, consisting of tetrahedral aluminium centers sharing bridging hydride ligands.DIBAL is useful in organic synthesis for a variety of reductions, including converting carboxylic acids, their derivatives, and nitriles to aldehydes.Nevertheless, it is possible to avoid these unwanted byproducts through careful control of the reaction conditions using continuous flow chemistry.[6] DIBAL, like most alkylaluminium compounds, reacts violently with air and water, potentially leading to explosion.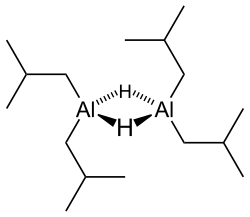
IUPAC nameCAS NumberChemSpiderECHA InfoCardEC NumberPubChemCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterSolubilityOccupational safety and healthGHS labellingPictogramsHazard statementsPrecautionary statementsstandard statereducing agentformulaisobutylorganoaluminium compoundempirical formulaX-ray crystallographyhydrideligandstriisobutylaluminiumβ-hydride elimination(CH3)2C=CH2toluenehexaneelectrophilicnucleophilicorganic synthesiscarboxylic acidsnitrilesaldehydesLiAlH4acyl chloridesalcoholsamineslactoneshemiacetalsflow chemistrycocatalystpolymerizationalkenesInorganica Chimica ActaZiegler, K.Justus Liebigs Annalen der ChemieAluminium compoundsOrganoaluminiumAl(C5(CH3)5)Al(BH4)3Al(CN)3NaAlCl4Al(NO3)3Al2(CO3)3Al(OH)3Al(OH)2OAcAl(OH)(OAc)2Al(OAc)3Al2SO4(OAc)4 Al(C5H7O2)3Al2(MoO4)3Al2(SO4)3Al2Se3Al2Te3Al2SiO5AlAsO4Al(OH)2CO2C17H5NaAlH2(OC2H4OCH3)2K2Al2B2O7K3AlF6(NH4)Al(SO4)2KAl(SO4)2NaAl(SO4)2Al(C3H5O3)3C36H69AlO6(Al(CH3)3)2(Al(C2H5)3)2Al(CH2CH(CH3)2)3Al(C2H5)2ClAl(C2H5)2CNAl(C2H5)2Cl2C2H5ClTi(C5H5)2CH2ClAl(CH3)2