Development of the nervous system
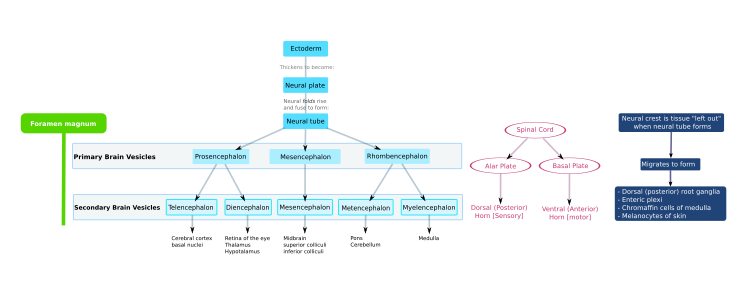
[9] As the embryo develops, the anterior part of the neural tube expands and forms three primary brain vesicles, which become the forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon).Embryonic cerebrospinal fluid differs from that formed in later developmental stages, and from adult CSF; it influences the behavior of neural precursors.[9] Because the neural tube gives rise to the brain and spinal cord any mutations at this stage in development can lead to fatal deformities like anencephaly or lifelong disabilities like spina bifida.Synaptic communication between neurons leads to the establishment of functional neural circuits that mediate sensory and motor processing, and underlie behavior.During neural induction, noggin and chordin are produced by the dorsal mesoderm (notochord) and diffuse into the overlying ectoderm to inhibit the activity of BMP4.Inhibition of TGF-β and BMP (bone morphogenetic protein) signaling can efficiently induce neural tissue from pluripotent stem cells.Patterning occurs due to specific environmental conditions - different concentrations of signaling molecules The ventral half of the neural plate is controlled by the notochord, which acts as the 'organiser'.Floor plate-derived Shh subsequently signals to other cells in the neural tube, and is essential for proper specification of ventral neuron progenitor domains.Signals that control anteroposterior neural development include FGF and retinoic acid, which act in the hindbrain and spinal cord.[16] The hindbrain, for example, is patterned by Hox genes, which are expressed in overlapping domains along the anteroposterior axis under the control of retinoic acid.[19] Subsequent waves of neurons split the preplate by migrating along radial glial fibres to form the cortical plate.Each wave of migrating cells travel past their predecessors forming layers in an inside-out manner, meaning that the youngest neurons are the closest to the surface.One example of ongoing tangential migration in a mature organism, observed in some animals, is the rostral migratory stream connecting subventricular zone and olfactory bulb.Instead these multipolar cells express neuronal markers and extend multiple thin processes in various directions independently of the radial glial fibers.The neurotrophic hypothesis was formulated by Victor Hamburger and Rita Levi Montalcini based on studies of the developing nervous system.Victor Hamburger discovered that implanting an extra limb in the developing chick led to an increase in the number of spinal motor neurons.Later they used a connectomic approach, i.e., tracing out all the connections between motor neurons and muscle fibers, to characterize developmental synapse elimination on the level of a full circuit.Analysis confirmed the massive rewiring, 10-fold decrease in the number of synapses, that takes place as axons prune their motor units but add more synaptic areas at the NMJs with which they remain in contact.Neurons in culture develop synapses that are similar to those that form in vivo, suggesting that synaptogenic signals can function properly in vitro.[56] Retinotopic map refinement occurs in downstream visual targets in the brain-the superior colliculus (SC) and dorsal lateral geniculate nucleus (LGN).[60] In the auditory system, spontaneous activity is thought to be involved in tonotopic map formation by segregating cochlear neuron axons tuned to high and low frequencies.The connectome can be constructed from diffusion MRI data: the vertices of the graph correspond to anatomically labelled gray matter areas, and two such vertices, say u and v, are connected by an edge if the tractography phase of the data processing finds an axonal fiber that connects the two areas, corresponding to u and v. Numerous braingraphs, computed from the Human Connectome Project can be downloaded from the http://braingraph.org site.The surprising observation is that the appearance of the edges is far from random: it resembles a growing, complex structure, like a tree or a shrub (visualized on the animation on the left).In vivo, it is suggested that muscle fibres select the strongest neuron through a retrograde signal or that activity-dependent synapse elimination mechanisms determine the identity of the "winning" axon at a motor endplate.As of 2021, scientists mapped and compared the whole brains of eight C. elegans worms across their development on the neuronal level[67][68] and the complete wiring of a single mammalian muscle from birth to adulthood.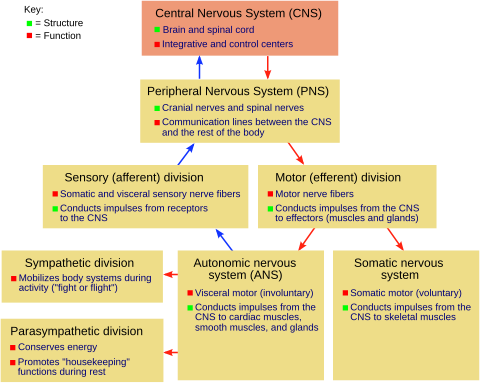

Development of the nervous system in humansorgan systemsDigestive systemReproductive systemUrinary systemEndocrine systemHuman developmentCirculatory systemnervous systemembryonic developmentneurosciencedevelopmental biologynematodesfruit fliesmammalsholoprosencephalyneurological disorderslimb paresisparalysisseizureshumansRett syndromeDown syndromeintellectual disabilityvertebratecentral nervous systemectodermgerm layerneuroectodermneural plateneural grooveneural tubeneurulationprimary brain vesiclesforebrainprosencephalonmidbrainmesencephalonhindbrainrhombencephalontelencephaloncerebral cortexbasal gangliadiencephalonthalamushypothalamuscolliculimetencephaloncerebellummyelencephalonmedullacentral canalspinal cordventricular systemcerebrospinal fluidanencephalyspina bifidaneural stem cellsneuronsglial cellsmigratedendritessynapsesneural circuitshuman brainepidermisneural ectodermmesodermendodermnotochordvertebral columnneural foldsbasal platealar plateneural canalexplantsXenopusbody plannogginchordinexplant culturesTGF-βpluripotent stem cellscephalic flexurecerebral hemispheresoptical vesiclechordatesfollistatinsonic hedgehogfloor platePatched1Smoothenedtranscription factorsmorphogeninterneuronsmotor neuronSr/Thr kinasesretinoic acidHox genesfacial nervetrigeminal nerveNeurogenesisprogenitor cellsEpigenetic modificationsgene expressionDNA cytosine methylation5-methylcytosine5-methylcytosine demethylationDNA methyltransferases (DNMTs)TET enzymes5-hydroxymethylcytosinebase excision repairCorticogenesisradial gliaCajal–Retzius cellsreelinmigrationtime-lapse microscopyganglionic eminenceventricular zoneneocortexradial glial cellpostmitoticsubplatemicrotubulecentrosome