Detergent
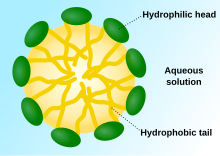
[3] Detergents are a group of compounds with an amphiphilic structure, where each molecule has a hydrophilic (polar) head and a long hydrophobic (non-polar) tail.In order to find alternatives for soap, synthetic detergents were made in Germany by chemists using raw material derived from coal tar.[15] The synthetic detergent created was more effective and less likely to form scum than soap in hard water, and can also eliminate acid and alkaline reactions and decompose dirt.Commercial detergent products with fatty alcohol sulphates began to be sold, initially in 1932 in Germany by Henkel.[15] In the United States, detergents were sold in 1933 by Procter & Gamble (Dreft) primarily in areas with hard water.[14] However, sales in the US grew slowly until the introduction of 'built' detergents with the addition of effective phosphate builder developed in the early 1940s.[14] The builder improves the performance of the surfactants by softening the water through the chelation of calcium and magnesium ions, helping to maintain an alkaline pH, as well as dispersing and keeping the soiling particles in solution.[14][18] They become incorporated in various products outside of laundry use, for example in dishwasher detergents, shampoo, toothpaste, industrial cleaners, and in lubricants and fuels to reduce or prevent the formation of sludge or deposits.[15][17] Developments over the years have included the use of enzymes, substitutes for phosphates such as zeolite A and NTA, TAED as bleach activator, sugar-based surfactants which are biodegradable and milder to skin, and other green friendly products, as well as changes to the form of delivery such as tablets, gels and pods.[23] Reagent grade detergents are employed for the isolation and purification of integral membrane proteins found in biological cells.

Detergent (disambiguation)surfactantmixturecleansingdilutesolutionsalkylbenzene sulfonateshard watersulfonatecarboxylatefatty acidlaundry detergentdish detergenthydrocarbonssteroidsurface tensionfoaming agentsmicellescritical micelle concentrationmonomeralkylbenzeneanionsalkyl groupsBile acidsdeoxycholic acidsodium dodecylbenzenesulfonatequaternary ammoniumpolyoxyethyleneglycosideTritonnonylphenoloctyl thioglucosidemaltosidesAmphotericzwitterionsSumerianancient EgyptSodium silicateHenkelsodium perboratePersilFirst World Warsulfationfatty alcoholProcter & Gamblephosphate builderchelationlaundry detergentsdishwasher detergentsbleachphosphates in detergentnutrient pollutionenzymeszeolitebleach activatorgreen friendlyLaundry detergent podsDishwasher detergentdish washinglaundryinternal combustion enginesfoulingaminesamidespolyisobuteneaminesuccinimideReagent gradeintegral membrane proteinsbiological cellscell membraneion channelslipopolysaccharidetransporterssignaling receptorsphotosystem IICleavable detergentDishwashing liquidDispersantGreen cleaningHard-surface cleanerList of cleaning productsTriton X-100Proceedings of the National Academy of Sciences of the United States of AmericaBibcodeBiophysical JournalWayback Machine
