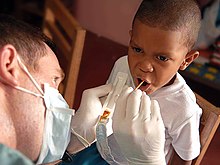
Biological aspects of fluorine
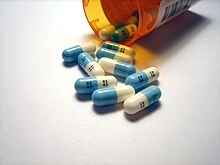
Though elemental fluorine (F2) is very rare in everyday life, fluorine-containing compounds such as fluorite occur naturally as minerals.Aside from their use in medicine, man-made fluorinated compounds have also played a role in several noteworthy environmental concerns.Chlorofluorocarbons (CFCs), once major components of numerous commercial aerosol products, have proven damaging to Earth's ozone layer and resulted in the wide-reaching Montreal Protocol; though in truth the chlorine in CFCs is the destructive actor, fluorine is an important part of these molecules because it makes them very stable and long-lived.Long-lived molecules from waterproofing sprays, for example PFOA and PFOS, are found worldwide in the tissues of wildlife and humans, including newborn children.Organofluorine in the form of its radioisotope 18F is also at the heart of a modern medical imaging technique known as positron emission tomography (PET).A PET scan produces three-dimensional colored images of parts of the body that use a lot of sugar, particularly the brain or tumors.Current thinking is that fluoride prevents cavities primarily by helping teeth that are in the very early stages of tooth decay.[6] Although the best available evidence shows no association with adverse effects other than fluorosis (dental and, in worse cases, skeletal), most of which is mild,[7] water fluoridation has been contentious for ethical, safety, and efficacy reasons,[6] and opposition to water fluoridation exists despite its widespread support by public health organizations.[14] Although the potential of fluorine being released in a fluoride leaving group depends on its position in the molecule,[15] organofluorides are generally very stable, since the carbon–fluorine bond is strong.The first fluorinated anesthetic agent, halothane, proved to be much safer (neither explosive nor flammable) and longer-lasting than those previously used.[26] In addition, inert fluorinated gases have the potential to be a cheap and efficient tool for imaging lung ventilation.[33] One effort, by Alliance Pharmaceuticals, reached clinical trials but was abandoned because of insufficient advantage compared to other therapies.It is used as a defense against herbivores by at least 40 green plants in Australia, Brazil, and Africa;[43] other biologically synthesized organofluorines include ω-fluoro fatty acids, fluoroacetone, and 2-fluorocitrate.[51] Fluoride is considered a semi-essential element for humans: not necessary to sustain life, but contributing (within narrow limits of daily intake) to dental health and bone strength.Above a concentration of 25 ppm, it causes significant irritation while attacking the eyes, airways and lungs and affecting the liver and kidneys.The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 0.1 ppm (0.2 mg/m3) over an 8-hour workday.Owing to its lesser chemical dissociation in water (remaining a neutral molecule), hydrogen fluoride penetrates tissue more quickly than typical acids.[60] Once in the blood, hydrogen fluoride reacts with calcium and magnesium, resulting in electrolyte imbalances, potentially including hypocalcemia.[62] Symptoms of exposure to hydrofluoric acid may not be immediately evident, with an eight-hour delay for 50% HF and up to 24 hours for lower concentrations.If the burn has been initially noticed, then HF should be washed off with a forceful stream of water for ten to fifteen minutes to prevent its further penetration into the body.Skin burns can be treated with a water wash and 2.5 percent calcium gluconate gel[64][65] or special rinsing solutions.[69] Chronic excess fluoride consumption can lead to skeletal fluorosis, a disease of the bones that affects millions in Asia and Africa.[69] Malfunction of water fluoridation equipment has occurred several times, including an Alaskan incident that sickened nearly 300 people and killed one.These compounds can enter the environment from their direct uses in waterproofing treatments and firefighting foams or indirectly from leaks from fluoropolymer production plants (where they are intermediates).[75][76][77] Trace quantities of PFCs have been detected worldwide, in organisms from polar bears in the Arctic to the global human population.A 2013 review showed widely varying amounts of PFOS and PFOA in different soils and groundwater, with no clear pattern of one chemical dominating.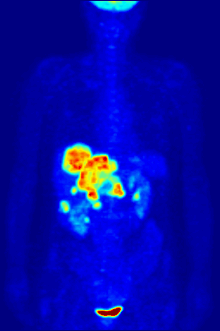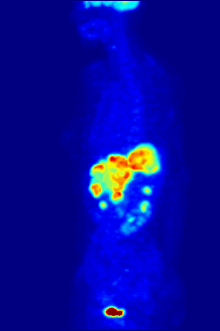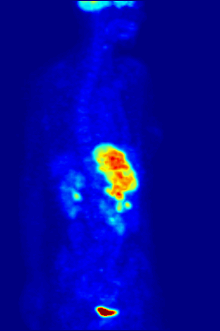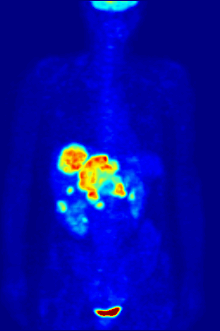








FluorinefluoriteorganofluorineLipitorProzacChlorofluorocarbonsozone layerMontreal Protocolchlorineorganofluorine compoundsbiopersistenceperfluorocarbonsliquid breathingpositron emission tomographyFluoride therapyWater fluoridationtooth decaytooth enamelhydroxyapatitecariescalciumtopical treatmentpublic water supplydentalskeletalopposition to water fluoridationpublic healthSystematic reviewsSodium fluoridetin difluoridesodium monofluorophosphatetoothpastemouthwashesdrug designcarbon–fluorine bondmetabolismhalf-livesaromatic ringepoxidesenzymeslipophilicitycarbon–hydrogen bondbioavailabilityleaving groupmineralocorticoidsblood pressurefludrocortisoneDexamethasonetriamcinolonecorticosteroidanesthetichalothanesevofluranedesfluraneenfluraneisofluranehydrofluorocarbonantidepressantsserotoninnorepinephrineselective serotonin reuptake inhibitorCelexaLexaproFluoroquinolonesbroad-spectrum antibioticsFludeoxyglucose (18F)positronsradiopharmaceutical2-deoxy-2-(18F)fluoro-D-glucoseTomographyassisted by a computerHodgkin's lymphomafluorine-19nuclear magnetic resonancenuclear spinnuclear magnetic momentmagnetogyric ratiorelaxationsignal-to-noise ratiohydrogen-1Blood substitutefluorocarbonsOxycyteMauro GianettiThe Abyssagrichemical compoundsSodium fluoroacetateKrebs cycleTrifluralingifblaarfluoroacetatedefense against herbivoresfatty acidsfluoroacetoneadenosyl-fluoride synthasefluoride deficiencyNFPA 704Occupational Safety and Health AdministrationPermissible exposure limitNational Institute for Occupational Safety and Healthrecommended exposure limitimmediately dangerous to life and healthChemical burnHydrofluoric acidmineral acidsnitric acidsulfuric acidhydrochloric acidhypocalcemiacardiac arrhythmiacalcium fluoridecalcium gluconatecalcium chlorideFluoride toxicityskeletal fluorosisexcretedPerfluorinated compoundPerfluorinated compoundspersistent global contaminantsperfluorooctanesulfonic acidperfluorooctanoic acidserum albuminlipophilicgenotoxicBottlenose dolphinsPPAR-alpha