Benzilic acid
Benzilic acid is an organic compound with formula C14H12O3 or (C6H5)2(HO)C(COOH).It is a white crystalline aromatic acid, soluble in many primary alcohols.[2] Benzilic acid is used in the manufacture of glycollate pharmaceuticals including clidinium, dilantin, flutropium, and mepenzolate which are antagonists of the muscarinic acetylcholine receptors.It is used in manufacture of the incapacitating agent 3-quinuclidinyl benzilate (BZ) which is regulated by the Chemical Weapons Convention.It is also monitored by law enforcement agencies of many countries, because of its use in the manufacture in hallucinogenic drugs.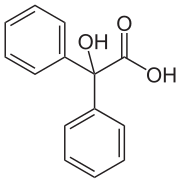

benzyl alcoholPreferred IUPAC nameCAS NumberBeilstein ReferenceChEMBLChemSpiderECHA InfoCardEC NumberGmelin ReferencePubChemCompTox DashboardSMILESChemical formulaMolar massDensityMelting pointBoiling pointSolubility in waterGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704standard stateorganic compoundaromaticprimary alcoholsbenzilethanolpotassium hydroxideLiebigdimerizationbenzaldehydebenzilic acid rearrangementglycollateclidiniumdilantinmepenzolateantagonistsmuscarinic acetylcholine receptorsincapacitating agent3-quinuclidinyl benzilateChemical Weapons Conventionhallucinogenic drugsThe Royal Society of ChemistryLiebig, J.Annalen der Chemie