Arsenic acid
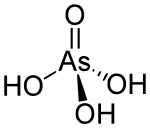
Arsenic acid as such has not been isolated, but is only found in solution, where it is largely ionized.[3] It is a tetrahedral species of idealized symmetry C3v with As–O bond lengths ranging from 1.66 to 1.71 Å.[5] The resulting solution is cooled to give colourless crystals of the hemihydrate H3AsO4·0.5H2O (or 2H3AsO4·H2O), although the dihydrate H3AsO4·2H2O is produced when crystallisation occurs at lower temperatures.[5] Arsenic acid is slowly formed when arsenic pentoxide is dissolved in water, and when meta- or pyroarsenic acid (H4As2O7) is treated with cold water.It has found occasional use as a wood preservative, a broad-spectrum biocide, a finishing agent for glass and metal, and a reagent in the synthesis of some dyestuffs and organic arsenic compounds.
IUPAC nameCAS NumberChEMBLChemSpiderECHA InfoCardEC NumberPubChemRTECS numberUN numberCompTox DashboardSMILESChemical formulaMolar masshygroscopicDensityMelting pointBoiling pointSolubility in waterSolubilityethanolVapor pressureAcidityConjugate baseArsenateMolecular shapeTetrahedralarsenicOccupational safety and healthGHS labellingPictogramsHazard statementsPrecautionary statementsNFPA 704Flash pointcationsSodium arsenateArsenous acidArsenic pentoxidePhosphoric acidstandard stateGreat Exhibitionchemical compoundformulaphosphatehemihydratesymmetrytriproticarsenate ioniodideiodinenitric acidDinitrogen trioxidewood preservativebiocidereagentdyestuffscorrosiveActa Crystallographica CHydrogen compoundsH3AsO3H[BF4]H2CrO4/H2Cr2O7H2MnO4H2MoO4NaHCO3H2N2O2H3NSO3H4P2O7H5P3O10H2[PtCl6]H2SeO3H2SeO4H4SiO4H2[SiF6]H2S2O3H2S2O6H2S2O7H2S2O8CF3SO3HH2TeO3H6TeO6H4TiO4H[Co(CO)4]